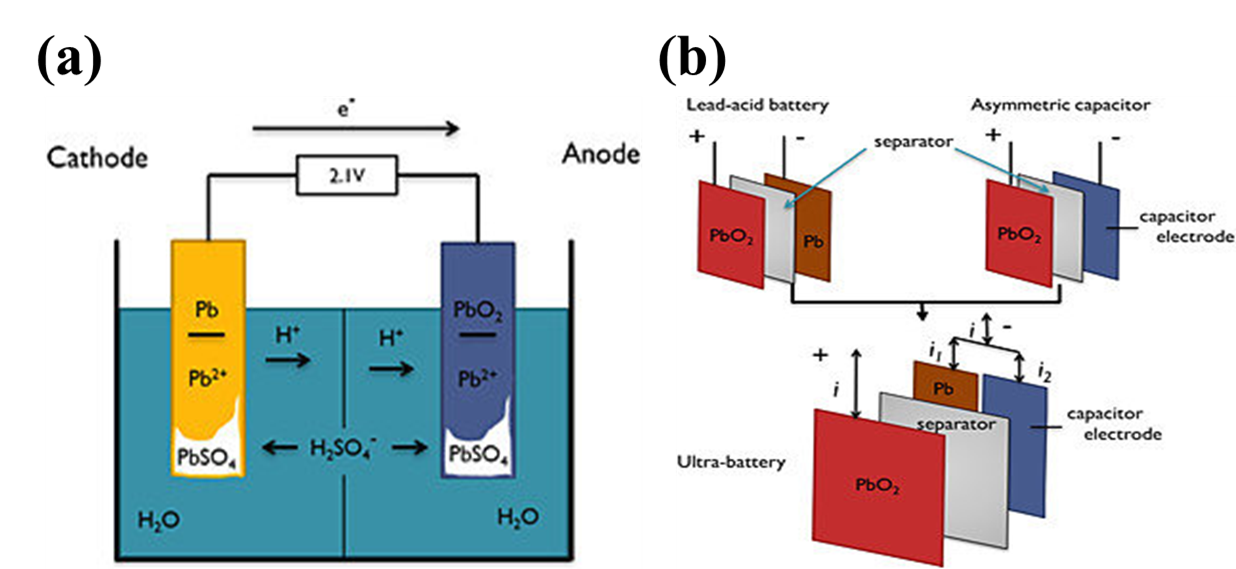
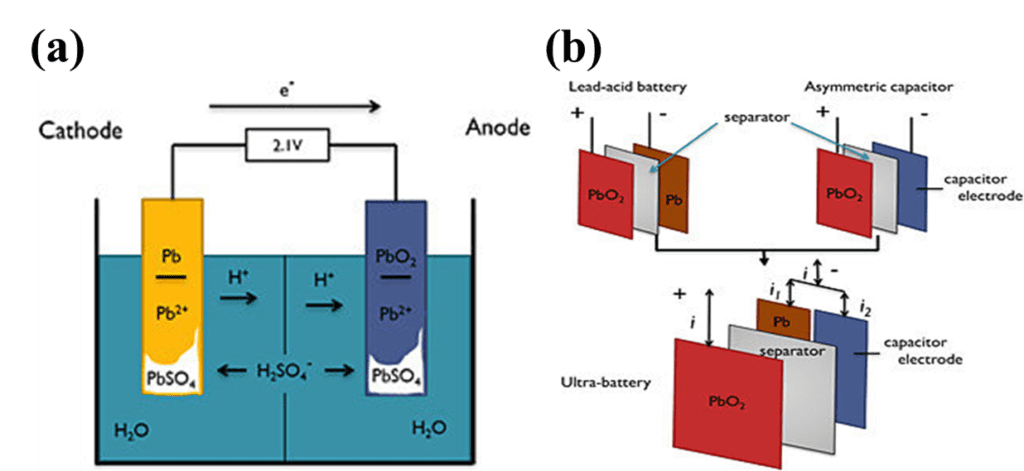
Lead-acid batteries are one of the oldest and most widely used types of rechargeable batteries in the world. They were invented in 1859 by the French engineer Gaston Planté and have since become an essential component in a wide range of applications, from starting cars and trucks to providing backup power for telecommunications and computer systems.
A lead-acid battery is made up of several individual cells, each consisting of two lead plates immersed in an electrolyte solution of sulfuric acid and water. One plate is made of pure lead, while the other is coated with lead dioxide. When the battery is charged, a chemical reaction takes place between the two plates and the electrolyte solution, converting the lead and lead dioxide into lead sulfate. When the battery is discharged, the reverse reaction occurs, converting the lead sulfate back into lead and lead dioxide and releasing electrical energy in the process.
One of the key advantages of lead-acid batteries is their relatively low cost compared to other types of rechargeable batteries. This is due in part to the fact that lead is a common and relatively inexpensive material, and that the manufacturing process for lead-acid batteries is well-established and highly automated. In addition, lead-acid batteries have a high energy density, meaning that they can store a large amount of energy in a relatively small space, making them well-suited for use in applications where space is at a premium.
However, lead-acid batteries also have a number of drawbacks. One of the main issues is that they are relatively heavy and bulky compared to other types of batteries, making them less suitable for portable applications such as laptops and mobile phones. In addition, lead-acid batteries have a relatively short lifespan compared to other types of batteries, typically lasting only a few years before they need to be replaced.
Another key issue with lead-acid batteries is that they require regular maintenance in order to ensure that they perform optimally. This includes topping up the electrolyte solution with distilled water, cleaning the battery terminals, and checking the battery’s voltage and state of charge on a regular basis. Failure to perform these maintenance tasks can lead to a range of problems, including reduced performance, shortened battery life, and even the risk of the battery exploding or catching fire.
Despite these issues, lead-acid batteries remain an essential component in many applications, particularly those where cost and reliability are the most important factors. They are widely used in the automotive industry for starting engines and powering accessories such as lights and radios, as well as in backup power systems for telecommunications and computer networks. In addition, lead-acid batteries are commonly used in renewable energy systems such as solar and wind power, where they are used to store excess energy generated during times of peak production for use during periods of low production.
In recent years, there has been increasing interest in developing new types of lead-acid batteries that are more efficient, longer-lasting, and more environmentally friendly. One approach that has shown promise is the use of advanced materials such as carbon additives and lead alloys to improve the performance and durability of lead-acid batteries. Another approach is the development of new manufacturing processes that use less energy and produce fewer emissions than traditional methods.
In conclusion, lead-acid batteries are an important and widely used type of rechargeable battery that has been in use for over a century. They offer a number of advantages, including low cost and high energy density, but also have a number of drawbacks, including relatively short lifespan and the need for regular maintenance. Despite these issues, lead-acid batteries continue to be used in a wide range of applications, and efforts are underway to develop new and improved versions of this important technology.
How Does a Lead-Acid Battery Work
A lead-acid battery works by converting chemical energy into electrical energy. The battery consists of several individual cells, each containing two lead plates immersed in an electrolyte solution of sulfuric acid and water.
When the battery is fully charged, the positive plate is coated with lead dioxide (PbO2), and the negative plate is pure lead (Pb). The electrolyte solution is mostly water (H2O) with sulfuric acid (H2SO4) dissolved in it.
During discharge, the sulfuric acid in the electrolyte reacts with the lead and lead dioxide on the plates, forming lead sulfate (PbSO4) on both plates and releasing electrons. The electrons flow out of the battery through the negative plate, through the external circuit, and back into the battery through the positive plate. This flow of electrons creates electrical energy that can be used to power devices.
During charging, an external electrical source is connected to the battery. The electrical energy from the source causes the reverse reaction to occur, converting the lead sulfate back into lead and lead dioxide on the plates and releasing hydrogen gas and oxygen gas from the electrolyte solution. The lead sulfate on the plates is broken down into lead and lead dioxide, and the hydrogen and oxygen gases are released from the electrolyte solution.
The charging process also causes the electrolyte solution to become more diluted, as water molecules are broken down into hydrogen and oxygen gases. To compensate for this, distilled water must be added to the electrolyte solution periodically to maintain the proper concentration of sulfuric acid.
The chemical reactions that take place in a lead-acid battery produce heat, which can cause the battery to become hot during use or charging. If the battery becomes too hot, it can be damaged or even explode. To prevent this, lead-acid batteries are often equipped with safety valves that release excess gas and pressure to prevent the buildup of heat.
Lead-acid batteries have a limited lifespan, typically lasting only a few years before they need to be replaced. This is because the repeated charging and discharging of the battery causes the lead plates to gradually degrade, reducing the battery’s capacity to store and deliver electrical energy.
In summary, a lead-acid battery works by converting chemical energy into electrical energy through a series of chemical reactions that take place between the lead plates and the electrolyte solution. During discharge, the lead sulfate is formed on both plates, releasing electrons that flow through the external circuit and back into the battery. During charging, the lead sulfate is converted back into lead and lead dioxide on the plates, releasing hydrogen and oxygen gases from the electrolyte solution. The battery’s limited lifespan is due to the gradual degradation of the lead plates caused by repeated charging and discharging.
Advantages and Benefits Lead-Acid Battery
Lead-acid batteries have been used for many decades and continue to be a popular choice for a variety of applications due to their advantages and benefits:
- Low cost: One of the primary advantages of lead-acid batteries is their low cost compared to other types of batteries. This makes them an affordable choice for many applications, including automotive, industrial, and backup power systems.
- High energy density: Lead-acid batteries have a relatively high energy density, which means they can store a large amount of energy in a relatively small space. This makes them a practical choice for applications where space is limited.
- Reliable: Lead-acid batteries are highly reliable and can provide consistent power over their lifespan. This is important for applications where uninterrupted power is critical, such as backup power systems or emergency lighting.
- Easy to maintain: Lead-acid batteries are easy to maintain, as they require only periodic checks of the electrolyte levels and occasional topping up with distilled water. This makes them a practical choice for applications where maintenance may be challenging, such as in remote or inaccessible locations.
- Wide temperature range: Lead-acid batteries can operate in a wide range of temperatures, from below-freezing to high temperatures. This makes them suitable for use in extreme environments, such as in automotive applications or outdoor equipment.
- Recyclable: Lead-acid batteries are highly recyclable, with up to 99% of their materials being recyclable. This makes them an environmentally friendly choice, as they can be reused or recycled at the end of their lifespan.
- High discharge rate: Lead-acid batteries can deliver high current outputs, making them suitable for applications where high power output is needed, such as starter motors or power tools.
- Widely available: Lead-acid batteries are widely available and can be found in most places around the world. This makes them a convenient choice for applications where replacement batteries may need to be obtained quickly.
- Established technology: Lead-acid battery technology has been around for many years and is well-established. This means that there is a wealth of knowledge and expertise available to help with troubleshooting and maintenance.
- Safety: Lead-acid batteries are relatively safe to use, as long as they are properly maintained and used in accordance with their specifications. They are also less prone to thermal runaways or fires than some other types of batteries, making them a practical choice for many applications.
In summary, lead-acid batteries offer many advantages and benefits, including low cost, high energy density, reliability, ease of maintenance, wide temperature range, recyclability, high discharge rate, availability, established technology, and safety. These benefits make lead-acid batteries a practical choice for many applications, including automotive, industrial, and backup power systems.
Disadvantages of lead-acid batteries
Lead-acid batteries have been in use for over 150 years and are widely used in a variety of applications, including automobiles, boats, and backup power systems. Despite their popularity, they also have several disadvantages that limit their usefulness in certain applications. In this response, I will outline some of the main disadvantages of lead-acid batteries.
- Limited lifespan: Lead-acid batteries have a limited lifespan, typically ranging from three to five years, depending on usage and maintenance. After this time, the battery’s performance begins to decline, and it will eventually need to be replaced.
- Heavy and bulky: Lead-acid batteries are heavy and bulky, making them unsuitable for portable applications. This is especially true for larger batteries used in backup power systems, which can weigh hundreds of pounds.
- Slow recharge time: Lead-acid batteries have a relatively slow recharge time, which can be a significant disadvantage in applications where quick recharging is necessary. This is because the battery chemistry requires a slow, controlled charge to avoid damaging the cells.
- Low energy density: Lead-acid batteries have a relatively low energy density compared to other battery types, such as lithium-ion. This means that they can store less energy per unit of weight or volume, making them less efficient for some applications.
- Maintenance requirements: Lead-acid batteries require regular maintenance to ensure that they operate correctly and safely. This includes checking the electrolyte level, cleaning the battery terminals, and equalizing the cells periodically.
- Environmental concerns: Lead-acid batteries contain lead and sulfuric acid, which are hazardous materials that can cause environmental damage if not disposed of properly. This is especially true for large-scale applications, such as backup power systems, where large numbers of batteries may need to be replaced at once.
- Limited temperature range: Lead-acid batteries have a limited temperature range in which they can operate effectively. Extreme temperatures can cause the battery’s performance to decline, and in some cases, can even damage the cells.
- Voltage drops: Lead-acid batteries experience voltage drops as they discharge, which can limit their usefulness in some applications. For example, in applications where a constant voltage is required, such as in some electronic devices, a lead-acid battery may not be suitable.
In conclusion, while lead-acid batteries have been a popular choice for many applications, they also have several disadvantages that limit their usefulness in certain situations. These include limited lifespan, heavy and bulky design, slow recharge time, low energy density, maintenance requirements, environmental concerns, limited temperature range, and voltage drops. When selecting a battery for a specific application, it is important to consider these factors and choose a battery that best meets the requirements of the application.
Present status and future of lead-acid batteries
Lead-acid batteries have been a popular choice for energy storage for over a century, and they continue to be used in a wide range of applications. While the development of new battery technologies, such as lithium-ion, has led some to question the future of lead-acid batteries, they still have a significant role to play in many industries. In this response, I will discuss the current status and future of lead-acid batteries.
Current Status:
Lead-acid batteries are still widely used in several industries, including:
- Automotive: Lead-acid batteries are the most common type of battery used in vehicles today. They are reliable, cost-effective, and can handle the high power demands of starting a vehicle’s engine.
- Backup power systems: Lead-acid batteries are often used in backup power systems, such as those found in data centers and hospitals. They provide reliable, long-lasting power when the primary power source fails.
- Renewable energy: Lead-acid batteries are also used in renewable energy systems, such as solar and wind power. They are cost-effective and can store energy for use when the sun is not shining or the wind is not blowing.
- Industrial: Lead-acid batteries are used in a wide range of industrial applications, such as forklifts, mining equipment, and golf carts.
Future:
While lead-acid batteries may face competition from newer battery technologies, such as lithium-ion, they still have a future in several applications. Some of the future developments of lead-acid batteries include:
- Improved energy density: Lead-acid batteries are expected to become more efficient and have higher energy densities, which will make them more competitive with other battery technologies.
- Advanced manufacturing techniques: Advances in manufacturing techniques, such as automated assembly lines and advanced materials, will help reduce the cost of lead-acid batteries and make them more affordable.
- Environmental concerns: As environmental concerns become more important, there is a focus on developing more environmentally friendly lead-acid batteries. This includes using recycled materials, improving the recycling process, and reducing the environmental impact of battery production and disposal.
- Emerging markets: There is a growing demand for lead-acid batteries in emerging markets, particularly in developing countries where access to reliable power is limited.
Conclusion:
Lead-acid batteries have been a reliable source of energy storage for over a century, and they continue to be used in a wide range of applications. While they face competition from newer battery technologies, they still have a future in several industries. As the demand for clean energy and sustainable technologies grows, lead-acid batteries are expected to become more efficient, cost-effective, and environmentally friendly.
lead acid Battery Lifetime
The lifetime of a lead-acid battery depends on several factors, including the type of battery, its usage pattern, and maintenance practices. Generally, a well-maintained lead-acid battery can last for several years.
The most common type of lead-acid battery is the flooded lead-acid battery, which has a lifespan of 3-5 years under normal usage conditions. However, with proper maintenance and care, the battery can last for up to 8 years.
Another type of lead-acid battery is the sealed lead-acid battery, which has a longer lifespan than the flooded type. Sealed lead-acid batteries can last for 5-7 years under normal usage conditions and up to 10 years with proper maintenance.
To maximize the lifespan of a lead-acid battery, it’s important to follow the manufacturer’s recommendations for charging and maintenance. This includes keeping the battery at the appropriate charge level, avoiding overcharging or deep discharging, and keeping the battery clean and free from corrosion. It’s also important to replace the battery if it shows signs of damage or wear, such as cracks, leaks, or reduced capacity.
Maintenance of lead acid Battery
Proper maintenance is essential to ensure the longevity and optimal performance of a lead-acid battery. Here are some tips for maintaining a lead-acid battery:
- Keep the battery clean and dry: Regularly inspect the battery for signs of corrosion or leaks. If you notice any corrosion or buildup around the terminals or case, clean it with a mixture of baking soda and water. Use a wire brush or steel wool to scrub away any rust or corrosion.
- Check the electrolyte level: For flooded lead-acid batteries, check the electrolyte level regularly and add distilled water if needed. The electrolyte level should be above the plates, but not overflowing.
- Avoid overcharging or deep discharging: Overcharging or deep discharging can damage the battery and reduce its lifespan. Use a charger that is specifically designed for lead-acid batteries, and avoid leaving the battery connected to the charger for too long.
- Store the battery properly: If you’re not using the battery for an extended period of time, store it in a cool, dry place. Make sure the battery is fully charged before storing it.
- Test the battery regularly: Use a battery tester or multimeter to check the voltage and overall health of the battery. If the battery is showing signs of reduced capacity or is not holding a charge, it may need to be replaced.
By following these maintenance tips, you can help ensure that your lead-acid battery lasts for as long as possible and continues to provide reliable power.
Special Considerations for Lead Acid Batteries
Lead-acid batteries are a type of rechargeable battery that has been used for more than 150 years. They are commonly used in applications where high power output is required, such as in vehicles, backup power systems, and renewable energy systems. While lead-acid batteries are known for their durability and reliability, there are some special considerations to keep in mind when using and maintaining these batteries.
- Charging: Proper charging is critical to the lifespan and performance of a lead-acid battery. Overcharging or undercharging can damage the battery and reduce its capacity. It’s important to use a charger that is specifically designed for lead-acid batteries and to follow the manufacturer’s recommendations for charging.
- Temperature: Lead-acid batteries perform best in moderate temperatures between 25-30°C (77-86°F). Extreme temperatures, both hot and cold, can reduce the battery’s capacity and lifespan. If the battery is operating in high temperatures, it’s important to monitor the electrolyte level regularly and add distilled water if needed.
- Ventilation: Lead-acid batteries produce hydrogen gas during charging, which can be explosive in high concentrations. It’s important to ensure that the battery is properly ventilated to prevent the buildup of hydrogen gas. If the battery is being used in an enclosed space, such as a vehicle or backup power system, make sure there is adequate ventilation to prevent the buildup of gas.
- Maintenance: Regular maintenance is essential to ensure the longevity and optimal performance of a lead-acid battery. This includes keeping the battery clean and dry, checking the electrolyte level, avoiding overcharging or deep discharging, storing the battery properly, and testing the battery regularly. Failure to properly maintain the battery can lead to reduced capacity and lifespan.
- Recycling: Lead-acid batteries are recyclable and should be properly disposed of when they reach the end of their lifespan. In many countries, there are regulations in place that require lead-acid batteries to be recycled. Recycling the battery not only prevents hazardous waste from entering the environment but also helps to conserve natural resources by reclaiming lead and other materials.
- Safety: Lead-acid batteries can be dangerous if not handled properly. Always wear protective gear, such as gloves and eye protection, when working with the battery. Avoid short-circuiting the battery, and never open or puncture the battery case.
In conclusion, lead-acid batteries are a reliable and durable power source, but they require special considerations to ensure optimal performance and longevity. Proper charging, temperature control, ventilation, maintenance, recycling, and safety precautions are all essential factors to keep in mind when using and maintaining lead-acid batteries. By following these guidelines, you can help ensure that your lead-acid battery provides reliable power for many years to come.