Introduction
Electrochemistry is a branch of science that explores the relationship between electrical energy and chemical reactions. It plays a crucial role in numerous applications, ranging from batteries and fuel cells to corrosion protection and electroplating. Understanding the basics of electrochemistry is fundamental for anyone interested in this field of study. In this comprehensive guide, we will delve into the key concepts, principles, and applications of electrochemistry.
I. Introduction to Electrochemistry
Electrochemistry is the study of electron transfer between chemical species and electrodes. It revolves around redox reactions, where one species loses electrons (oxidation) while another gains electrons (reduction). These electron transfers are facilitated by electrochemical cells, devices designed to harness and control the flow of electrons.
II. Redox Reactions and Half-Reactions
A. Oxidation and Reduction: In a redox reaction, one species undergoes oxidation, losing electrons, and another species undergoes reduction, gaining those electrons. This exchange is central to all electrochemical processes.
B. Half-Reactions: In electrochemistry, redox reactions are often split into two half-reactions – one for the oxidation process and one for the reduction process. These half-reactions help in understanding electron flow and balancing equations.
III. Electrochemical Cells
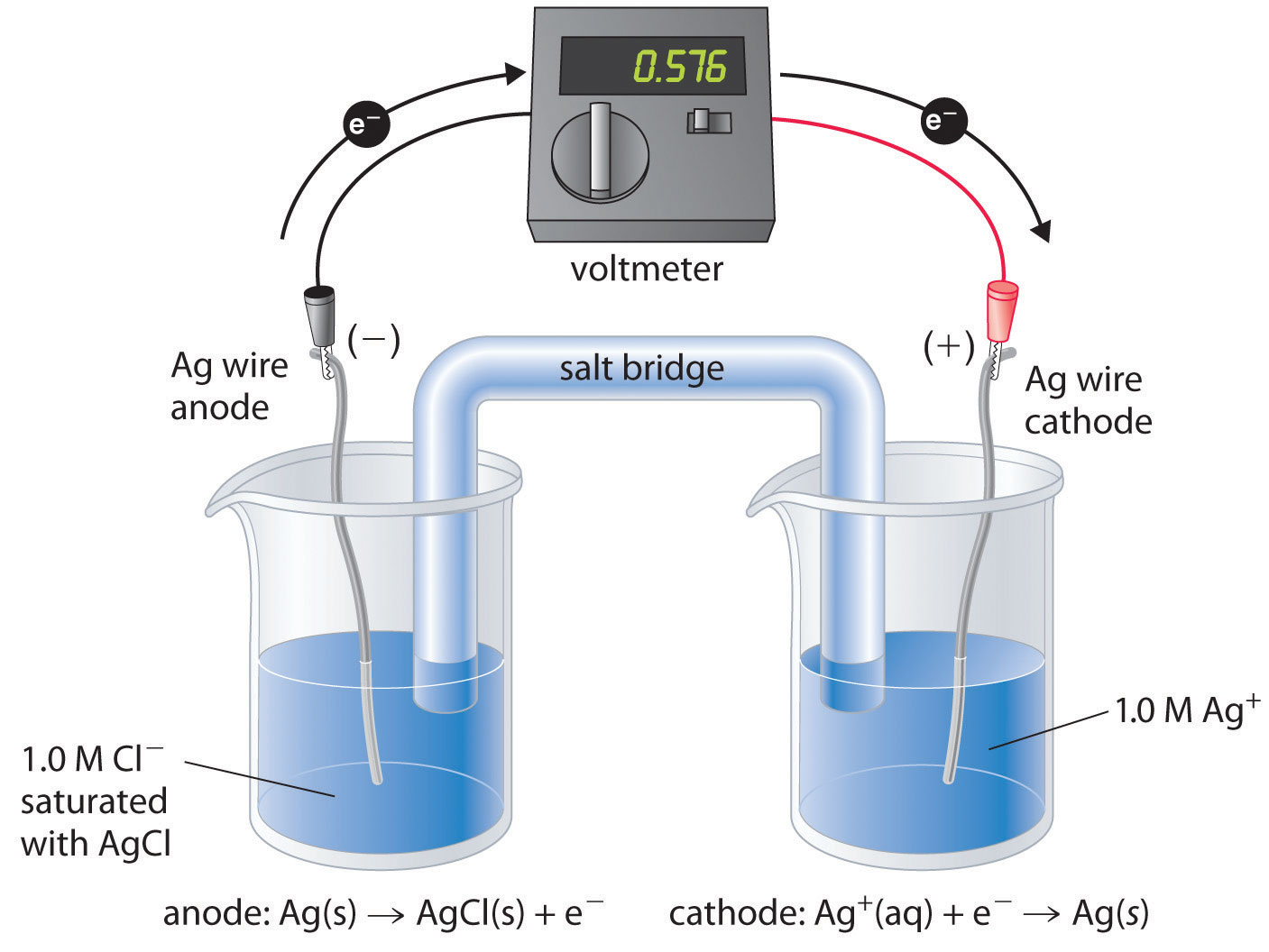
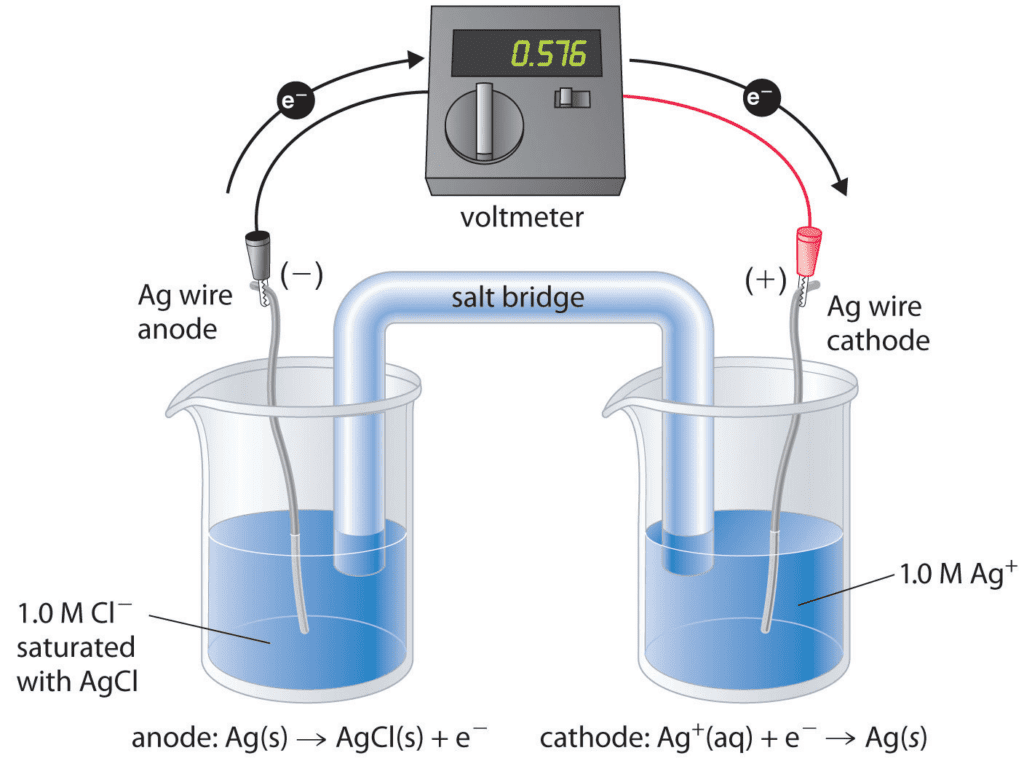
A. Galvanic Cells (Voltaic Cells): Galvanic cells convert chemical energy into electrical energy spontaneously. They consist of two half-cells connected by an external circuit. The half-reactions take place in their respective compartments, and electrons flow through the external circuit from the anode (site of oxidation) to the cathode (site of reduction).
B. Electrolytic Cells: In contrast to galvanic cells, electrolytic cells require an external source of electrical energy to drive non-spontaneous redox reactions. The flow of current causes the oxidation and reduction processes to occur, allowing for chemical transformations.
IV. Electrode Potentials and the Nernst Equation
A. Standard Electrode Potential: The standard electrode potential measures the tendency of an electrode to undergo reduction compared to a standard hydrogen electrode (SHE). It is a crucial parameter in predicting the direction of electron flow.
B. Nernst Equation: The Nernst equation relates the electrode potential of an electrochemical cell to the concentrations of reactants and products, temperature, and the Faraday constant. It allows us to calculate the cell potential under non-standard conditions.
V. Energy and Thermodynamics in Electrochemistry
A. Gibbs Free Energy: The change in Gibbs free energy (ΔG) determines the spontaneity of a redox reaction. If ΔG is negative, the reaction is spontaneous, and energy is released. For non-spontaneous reactions, an external energy source is required.
B. Relationship between Cell Potential and ΔG: The cell potential (Ecell) is related to ΔG through the equation ΔG = -nFEcell, where n is the number of electrons transferred, and F is the Faraday constant.
VI. Types of Electrodes
A. Inert Electrodes: Inert electrodes, like platinum, do not participate in the redox reaction but serve as conductive surfaces to facilitate electron transfer.
B. Reactive Electrodes: Reactive electrodes actively take part in the redox reaction. An example is the use of metal electrodes in certain electroplating processes.
VII. Electrochemical Series and Electrode Potential
A. Electrochemical Series: The electrochemical series ranks different metals and substances based on their standard electrode potentials. This series helps predict which species will be preferentially oxidized or reduced in a given electrochemical cell.
B. Predicting Spontaneity: Using the electrochemical series, we can determine if a redox reaction will occur spontaneously or if an external potential is needed.
VIII. Faraday’s Laws of Electrolysis
A. First Faraday’s Law: The amount of substance undergoing electrolysis is directly proportional to the quantity of electricity passed through the cell.
B. Second Faraday’s Law: When the same quantity of electricity passes through different electrolytes, the mass of the substance liberated or deposited at the electrodes is directly proportional to its equivalent weight.
IX. Applications of Electrochemistry
A. Batteries: Electrochemistry is the foundation of batteries, which store electrical energy for various applications, from powering portable electronics to storing renewable energy.
B. Fuel Cells: Fuel cells are electrochemical devices that convert chemical energy directly into electrical energy, with applications in vehicles and stationary power generation.
C. Corrosion Protection: Electrochemical methods, such as sacrificial anode cathodic protection, are used to protect metals from corrosion.
D. Electroplating: Electroplating is an electrochemical process used to coat a surface with a layer of metal for decorative or functional purposes.
E. Sensors and Biosensors: Electrochemical sensors and biosensors detect and quantify specific analytes, finding applications in environmental monitoring and medical diagnostics.
Remember, this comprehensive guide merely scratches the surface of electrochemistry’s depth and complexity. Continuously exploring this fascinating field will lead to a deeper appreciation of its vast potential and impact on our world.
Electrochemical Techniques
A. Cyclic Voltammetry: Cyclic voltammetry is a widely used electrochemical technique that measures the current response of an electrochemical cell to a range of voltage sweep rates. It provides valuable information about redox reactions and the electrochemical behavior of various substances.
B. Electrochemical Impedance Spectroscopy (EIS): EIS is a powerful technique that measures the impedance response of an electrochemical system to an applied small-amplitude sinusoidal voltage or current. It is particularly useful for studying electrode interfaces, corrosion processes, and electrochemical kinetics.
C. Potentiostatic and Galvanostatic Methods: potentiostatic and galvanostatic techniques involve applying a constant voltage or current to an electrochemical cell. These methods are essential for studying electrodeposition, corrosion studies, and fuel cell operation.
D. Chronoamperometry and Chronopotentiometry: These techniques involve applying a constant current or voltage step to an electrochemical cell and measuring the resulting time-dependent current or potential. They are used to study reaction kinetics and diffusion-controlled processes.
Advanced Topics in Electrochemistry
A. Nanoelectrochemistry: Nanoelectrochemistry explores the electrochemical behavior of nanomaterials, including nanoparticles and nanocomposites. This emerging field has applications in catalysis, energy storage, and sensing.
B. Photoelectrochemistry: Photoelectrochemistry investigates the interaction between light and electrochemical processes. It has significant implications for solar energy conversion, photoelectrochemical cells, and artificial photosynthesis.
C. Electrocatalysis: Electrocatalysis focuses on enhancing the rates of electrochemical reactions using catalysts. Efficient electrocatalysts are vital for renewable energy technologies and fuel cells.
D. Bioelectrochemistry: Bioelectrochemistry explores the electrochemical processes in biological systems, including redox reactions in enzymes and the electrical properties of living cells.
Cutting-Edge Research and Future Perspectives
A. Energy Storage Innovations: Researchers are exploring advanced materials and designs for batteries, supercapacitors, and other energy storage devices to enhance energy density and cycling performance.
B. Electrified Transport: The development of electrochemical technologies plays a key role in advancing electric vehicles, making them more efficient, affordable, and accessible.
C. Sustainable Fuel Production: Electrochemical processes, such as electrochemical reduction of CO2 (CO2RR), hold promise for producing sustainable fuels from renewable energy sources.
D. Smart Materials and Sensors: Electrochemical sensors and smart materials are continually evolving, paving the way for better environmental monitoring, healthcare diagnostics, and real-time data collection.
The Importance of Electrochemistry in Society
A. Green Energy Solutions: Electrochemistry enables the advancement of green energy solutions, contributing to a sustainable future with reduced dependence on fossil fuels.
B. Environmental Protection: Electrochemical technologies aid in wastewater treatment, pollution monitoring, and reducing the environmental impact of industrial processes.
C. Healthcare and Biomedical Applications: Electrochemical sensors and biosensors play a vital role in medical diagnostics, drug discovery, and personalized healthcare.
D. Industry and Technology Advancements: Electrochemistry underpins various industries, including electronics, aerospace, metallurgy, and chemical manufacturing, driving technological advancements worldwide.
Top 10 books on Basic Electrochemistry
Here is a list of top 10 books on Basic Electrochemistry:
- “Electrochemical Methods: Fundamentals and Applications” by Allen J. Bard and Larry R. Faulkner
- This book is considered the classic textbook on electrochemistry, covering fundamental concepts and techniques in a comprehensive manner.
- “Modern Electrochemistry 1: Ionics” by John O’M. Bockris and Amulya K.N. Reddy
- This book focuses on ionic processes, covering topics like electrochemical cells, ionic transport, and electrode processes.
- “Electrochemical Systems” by John Newman and Karen E. Thomas-Alyea
- This text provides an in-depth introduction to electrochemical systems and their applications, with clear explanations and examples.
- “Electrochemistry” by Carl H. Hamann, Andrew Hamnett, and Wolf Vielstich
- A widely-used textbook that covers the principles of electrochemistry and includes practical examples and applications.
- “Electrochemical Methods: Student Solutions Manual: Fundamentals and Applications” by Allen J. Bard and Larry R. Faulkner
- This is the solutions manual for the “Electrochemical Methods” textbook, providing answers to the exercises and problems.
- “Electrochemical Engineering” by Thomas F. Fuller, John Newman, and Jacek Nowotny
- This book covers the engineering aspects of electrochemistry, including electrochemical processes in energy conversion and storage.
- “Electrochemical Techniques in Corrosion Science and Engineering” by Robert White
- Focused on corrosion, this book explores the electrochemical techniques used to understand and prevent corrosion.
- “Electrochemical Methods: Student Solutions Manual: Fundamentals and Applications” by Allen J. Bard and Larry R. Faulkner
- This is the solutions manual for the “Electrochemical Methods” textbook, providing answers to the exercises and problems.
- “A Handbook of Electrochemical Engineering” by C. M. Schlesinger and A. J. McLean
- This handbook covers practical aspects of electrochemical engineering and is a valuable reference for professionals in the field.
- “Experimental Electrochemistry: A Laboratory Textbook” by Carl H. Hamann, Andrew Hamnett, and Wolf Vielstich
- This lab-focused book offers practical guidance on conducting electrochemical experiments.
Please note that there might be newer books or editions available beyond my last update, so it’s always a good idea to check for the latest publications and editions.
Conclusion
In conclusion, electrochemistry is an interdisciplinary field with far-reaching applications that continue to shape our world. From fundamental concepts like redox reactions and electrode potentials to cutting-edge research in nano electrochemistry and photoelectrochemical cells, the study of electrochemistry offers a wealth of opportunities for scientific exploration and innovation. As we look to the future, the continued progress in electrochemical research holds tremendous potential for addressing global challenges, promoting sustainable technologies, and advancing society as a whole. electrochemistry is a captivating field that underpins various technologies and applications essential to modern life. Understanding the basics of electrochemistry provides a solid foundation for further exploration into more advanced topics. From electrochemical cells to electrode potentials and the applications of this science, electrochemistry continues to drive innovations across industries and contributes significantly to scientific advancements.