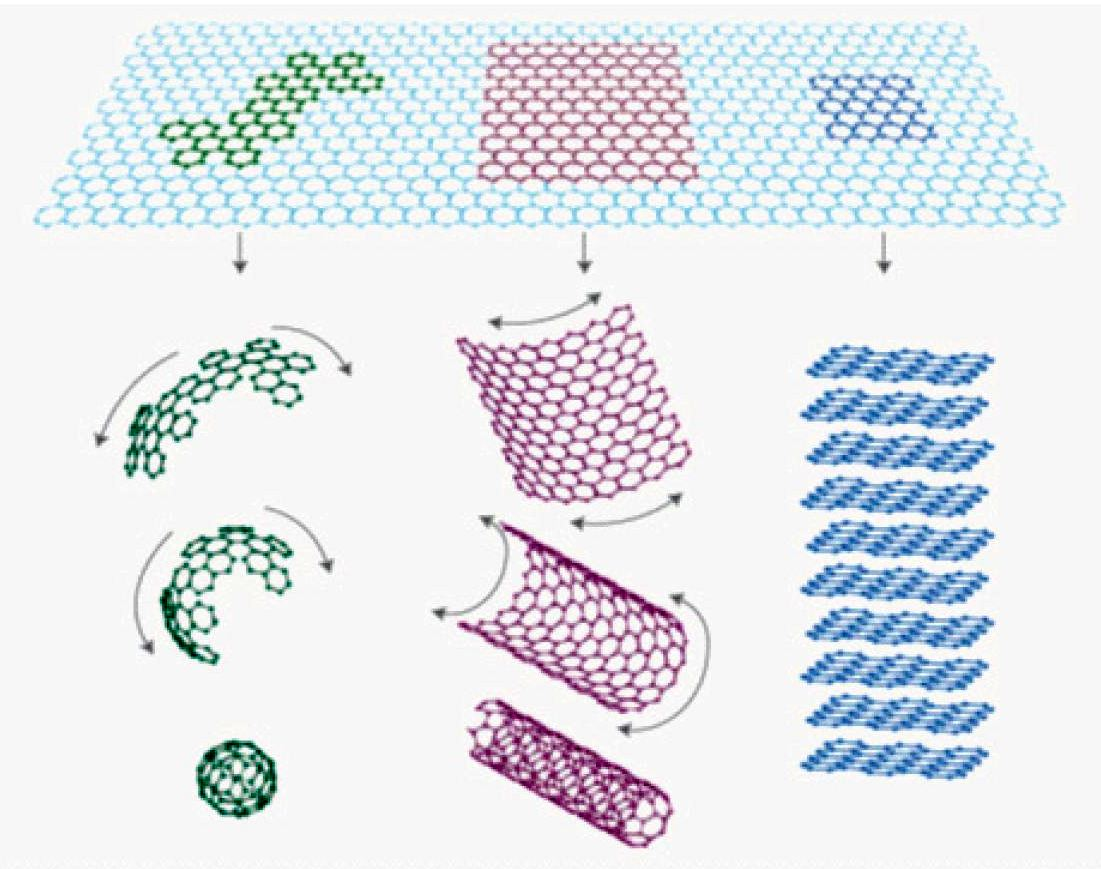
How Graphene Is Made
Introduction
Graphene is a two-dimensional allotrope of carbon that consists of a single layer of carbon atoms arranged in a hexagonal lattice. It was first isolated in 2004 by Andre Geim and Konstantin Novoselov, who were awarded the Nobel Prize in Physics in 2010 for their discovery.
Graphene has a wide range of unique mechanical, electrical, and thermal properties that make it a wonder material with numerous potential applications in various industries. It is the thinnest material known to exist and is incredibly strong, flexible, and transparent. It is also an excellent conductor of electricity and heat, with high surface area and chemical stability.
Graphene has potential applications in many areas, including electronics, energy storage, biomedicine, and aerospace. It can be used to make faster and more efficient transistors, sensors, and batteries, as well as lightweight and strong composites and coatings. It also has potential uses in drug delivery, water purification, and cancer therapy.
The unique properties of graphene arise from its two-dimensional structure, which allows for high electron mobility, quantum confinement, and strong interatomic bonding. Graphene also has a high surface-to-volume ratio, which makes it highly reactive and provides large surface areas for chemical reactions.
The production of graphene has evolved rapidly in the past few years, with various techniques and processes being developed to synthesize graphene in large quantities and with specific properties. Some of the most common methods for graphene synthesis include mechanical exfoliation, chemical vapor deposition, chemical reduction of graphene oxide, electrochemical exfoliation, and laser ablation.
Graphene is an exciting material with vast potential for future applications. Its unique properties and versatility make it a promising candidate for solving many of the challenges faced by modern industries, from developing new electronic devices to creating more sustainable energy storage systems.
There are various techniques and processes for making graphene, each with its own advantages and limitations. In this article, we will discuss some of the most common methods used for graphene synthesis.
Synthesis Strategies
Mechanical exfoliation
Mechanical exfoliation is a method for producing graphene that involves the manual or mechanical peeling of graphite layers from a bulk graphite crystal. This method was first used to isolate graphene in 2004 by Andre Geim and Konstantin Novoselov, who won the Nobel Prize in Physics for their work.
The mechanical exfoliation method for graphene production involves the following steps:
- Preparation of graphite: A high-quality graphite crystal is selected and prepared by cleaving it with tape to remove any surface impurities or defects.
- Peeling of graphene layers: A piece of the prepared graphite crystal is placed on a substrate, and a small piece of tape is gently pressed onto the surface of the graphite crystal. The tape is then pulled away from the crystal, taking some graphene layers with it.
- Transfer of graphene: The tape with the graphene layers is then transferred to a clean substrate, and the tape is gently pressed onto the substrate to transfer the graphene layers onto it.
- Repeated peeling: The peeling process is repeated multiple times to obtain a single-layer or few-layer graphene.
The quality and properties of graphene produced by mechanical exfoliation depend on several factors, such as the quality and thickness of the original graphite crystal, the choice of tape, and the peeling and transfer process. Mechanical exfoliation can produce high-quality, defect-free graphene with excellent electronic and mechanical properties.
However, this method is not scalable and is highly labor-intensive, making it unsuitable for large-scale production of graphene.
Chemical vapor deposition (CVD)
Chemical vapor deposition (CVD) is one of the most common and effective methods for synthesizing high-quality graphene in large quantities. The process involves the deposition of a hydrocarbon gas such as methane (CH4) onto a substrate at high temperatures in the presence of a metal catalyst.
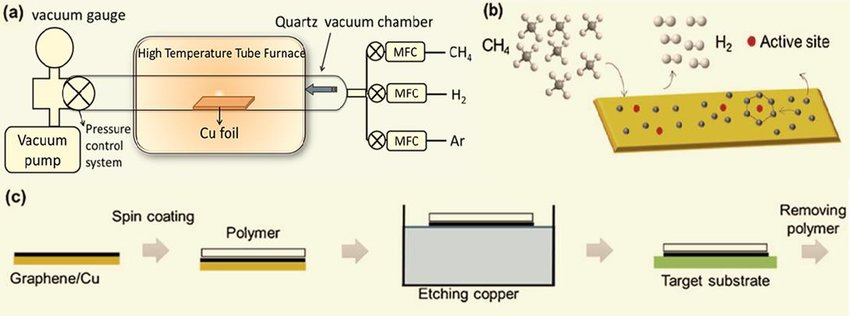
The CVD process for graphene production typically involves the following steps:
- Substrate preparation: The substrate, usually copper or nickel, is first cleaned to remove any impurities or surface contaminants that could interfere with graphene growth.
- Catalyst deposition: A thin layer of a metal catalyst, typically copper or nickel, is deposited onto the substrate using methods such as thermal evaporation, sputtering, or electrochemical deposition.
- Graphene growth: The substrate is then heated to high temperatures (typically around 1000°C) in the presence of a hydrocarbon gas such as methane. The hydrocarbon gas decomposes on the surface of the metal catalyst, leading to the growth of graphene.
- Cooling and transfer: After the graphene has grown to the desired thickness, the substrate is cooled down slowly to room temperature to prevent cracking or damage to the graphene. The graphene is then transferred to a different substrate or support material using methods such as wet transfer or dry transfer.
The quality and properties of the graphene produced by CVD depend on several factors, such as the type and quality of the substrate, the choice of hydrocarbon gas, the temperature and pressure conditions, and the duration of the growth process. The CVD method can produce high-quality, large-area graphene films with tunable properties and is scalable for commercial production.
The resulting graphene can be transferred onto a different substrate by using a polymer film or etching away the metal substrate.
CVD is a highly versatile technique that can produce high-quality graphene films with tunable properties. However, it requires specialized equipment and is relatively expensive compared to other methods.
Chemical reduction of graphene oxide
Graphene oxide (GO) reduction is a method for producing graphene that involves the reduction of GO to graphene using a reducing agent. GO is a derivative of graphene that is easier to make in large quantities. It is produced by oxidizing graphite using strong oxidizing agents such as potassium permanganate or nitric acid.
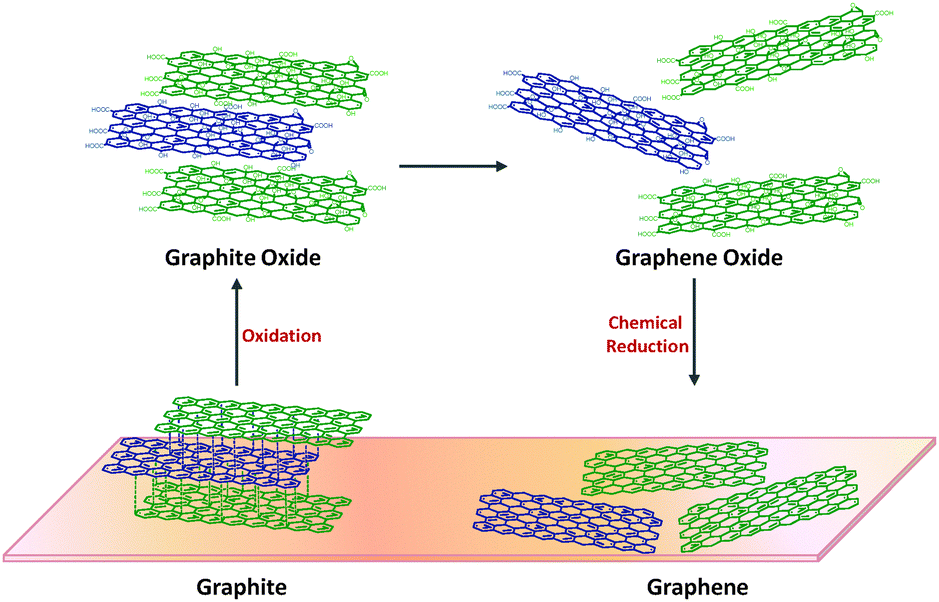
Image Credit:
Chem. Soc. Rev., 2014,43, 291-312
The chemical reduction of graphene oxide for graphene production involves the following steps:
- Preparation of GO: Graphene oxide is first prepared by oxidizing graphite using strong oxidizing agents such as potassium permanganate or nitric acid. This process introduces oxygen-containing functional groups on the graphene surface, making it hydrophilic and soluble in water.
- Reduction of GO: GO is then reduced back to graphene using a reducing agent such as hydrazine, sodium borohydride, or hydrogen. The reducing agent removes the oxygen-containing functional groups on the graphene surface, restoring the sp2 hybridization of the carbon atoms and converting GO to graphene.
- Washing and filtration: The reduced graphene is then washed several times with water or a solvent such as ethanol to remove any residual reducing agent and impurities. The graphene is then filtered and dried to obtain a powder or film.
The quality and properties of graphene produced by chemical reduction depend on several factors, such as the quality and thickness of the original graphene oxide, the choice of reducing agent, and the reduction process conditions. The chemical reduction can produce graphene with a high yield and tunable properties, making it suitable for large-scale production. However, it may introduce defects and impurities on the graphene surface, affecting its electronic and mechanical properties.
Electrochemical exfoliation
Electrochemical exfoliation is a method for producing graphene that involves the electrochemical reduction of graphene oxide (GO) in a solution using an electric field. This method offers a simple and scalable way to produce high-quality graphene with well-controlled properties.
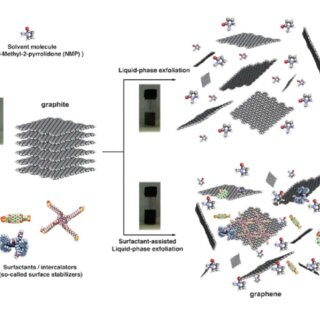
The electrochemical exfoliation method for graphene production involves the following steps:
- Preparation of GO: Graphene oxide is first prepared by oxidizing graphite using strong oxidizing agents such as potassium permanganate or nitric acid. This process introduces oxygen-containing functional groups on the graphene surface, making it hydrophilic and soluble in water.
- Electrochemical reduction: The GO solution is placed in an electrochemical cell with two electrodes, one as an anode and the other as a cathode. A direct current (DC) voltage is applied across the electrodes, causing the reduction of GO to graphene at the cathode. The oxygen-containing functional groups are removed, and the sp2 hybridization of the carbon atoms is restored, converting GO to graphene.
- Washing and filtration: The reduced graphene is then washed several times with water or a solvent such as ethanol to remove any residual reducing agent and impurities. The graphene is then filtered and dried to obtain a powder or film.
The quality and properties of graphene produced by electrochemical exfoliation depend on several factors, such as the quality and thickness of the original graphene oxide, the choice of electrode materials and electrolytes, the voltage and current conditions, and the reduction time. Electrochemical exfoliation can produce graphene with high quality, low defect density, and tunable properties, making it suitable for various applications. However, it requires careful control of the reduction conditions to avoid the formation of defects and impurities.
Laser ablation
Graphene is a two-dimensional material that has garnered a lot of attention in recent years due to its exceptional properties, such as high strength, high electrical and thermal conductivity, and large surface area. One of the methods used to synthesize graphene is laser ablation. In this method, a high-power laser is used to vaporize a target material, which is then deposited onto a substrate to form graphene.
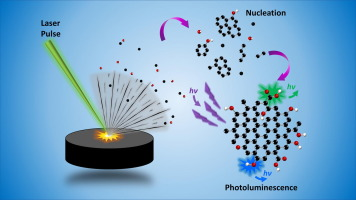
Photo Credit: Journal of Colloid and Interface Science, 527, 132-140.
The process of laser ablation involves several steps. Firstly, a high-power laser is directed at a target material, typically graphite or a metal foil. The laser rapidly heats up the target material, causing it to vaporize into a plasma. The plasma is composed of a mixture of ions, atoms, and free electrons. The laser also creates a shock wave that propagates through the plasma, causing the plasma to expand rapidly.
As the plasma expands, it cools down, and the atoms and ions start to recombine to form nanoparticles. These nanoparticles are composed of a few layers of graphene and are known as graphene flakes. The graphene flakes are suspended in a gas phase, and they can be deposited onto a substrate by various methods, such as electrostatic attraction, gravitational settling, or gas flow.
The quality of the graphene synthesized by laser ablation depends on several factors, such as the laser power, the target material, the substrate, and the deposition conditions. Higher laser power leads to higher temperatures and more energetic plasma, which can result in the formation of larger graphene flakes with fewer defects. The target material also plays a crucial role in determining the quality of the graphene. Graphite is a commonly used target material as it has a layered structure that facilitates the formation of graphene flakes. Metal foils can also be used as target materials, but they require additional processing steps to remove the metal impurities from the graphene.
The substrate used for deposition also affects the quality of the graphene. A smooth and clean substrate promotes the growth of high-quality graphene with fewer defects. The deposition conditions, such as the gas composition and pressure, also play a role in determining the quality of the graphene. For example, the presence of hydrogen gas during deposition can reduce the number of defects in the graphene by passivating the dangling bonds on the edges of the graphene flakes.
One of the advantages of laser ablation is that it allows for the synthesis of large-area graphene films with a high degree of control over the graphene thickness and quality. The size of the graphene flakes can be controlled by adjusting the laser power, and the number of layers in the graphene can be controlled by adjusting the deposition time. Laser ablation can also be used to pattern graphene into specific shapes and sizes, making it suitable for various applications.
Laser ablation is a relatively simple and scalable method for the synthesis of graphene, and it has the potential to be used for large-scale production of graphene. However, there are some challenges associated with this method. One of the main challenges is the formation of defects in the graphene, which can affect its properties. Defects can be introduced during the synthesis process or can be present in the starting material. Defects can also be introduced during the deposition process due to the interaction of the graphene flakes with the substrate or the gas phase.
Another challenge is the scalability of the method. While laser ablation is a scalable method, it is limited by the size of the laser and the target material. Large-area graphene films require large lasers and target materials, which can be expensive and difficult to handle. Therefore, alternative methods such as chemical vapor deposition (CVD) are being developed to address these challenges.
In conclusion, laser ablation is a promising method for the synthesis of graphene with a high degree of control over the graphene thickness and quality. However, there are some challenges associated with this method, such as the formation of defects and
Top Books on Graphene Synthesis
Here are 20 recommended books on Graphene synthesis:
- Graphene: Synthesis and Applications by Wonbong Choi and Zafar Iqbal
- Graphene: Fundamentals and Emergent Applications by Jamie H. Warner, Franziska Schaffel, and Mark Rummeli
- Graphene: Synthesis, Properties, and Phenomena by C.N.R. Rao, A.K. Sood, and U. Maitra
- Graphene Oxide: Fundamentals and Applications by A.K. Haghi, G.E. Zaikov, and A. Katashev
- Graphene-Based Materials in Health and Environment by Seyedeh Alieh Kazemi Abadi and Inamuddin
- Graphene: Energy Storage and Conversion Applications by A.K. Haghi, G.E. Zaikov, and M. Mohammadi
- Graphene Nanomaterials: Synthesis, Characterization, Properties, and Applications by C. N. R. Rao, Anindya Das, and G. U. Kulkarni
- Handbook of Graphene: Growth, Synthesis, and Functionalization by Eva Andrei
- Graphene Science Handbook: Applications and Industrialization by Mahmood Aliofkhazraei
- Graphene Science Handbook: Fabrication Methods by Mahmood Aliofkhazraei
- Graphene-Based Polymer Nanocomposites in Electronics by Kishor Kumar Sadasivuni, Deepalekshmi Ponnamma, and Mariam AlAli AlMaadeed
- Graphene: Properties, Preparation, Characterisation, and Devices by V. Georgakilas, J.N. Tiwari, K.C. Kemp, and N. A. Koratkar
- Graphene and its Fascinating Attributes by Madhuri Sharon and Neeraj Kumar Mishra
- Handbook of Graphene, Volume 1: Growth, Synthesis, and Functionalization by Eva Andrei
- Graphene for Transparent Conductors: Synthesis, Properties and Applications by Veena Choudhary
- Graphene and its Nanostructured Derivatives: Fabrication and Applications in Energy Storage and Conversion by Zhaoping Liu
- Graphene Nanoelectronics: Metrology, Synthesis, Properties, and Applications by Masataka Hasegawa
- Graphene-Based Materials for Energy Conversion by M.A. Shah, I. Al-Omari, and H.M. Ang
- Graphene: The Superstrong, Superthin, and Superversatile Material That Will Revolutionize the World by Les Johnson and Joseph E. Meany
- Graphene: Carbon in Two Dimensions by M. F. Craciun and S. Russo
These books cover a range of topics related to Graphene synthesis, including properties, applications, and fabrication methods, among others.
World’s’s top 10 companies for mass production of Graphene
It is difficult to identify the top 10 companies for mass production of graphene as the industry is still in its early stages and the market is highly fragmented. However, here are some of the companies that are known for their graphene production capabilities:
- Haydale Graphene Industries: Based in the UK, Haydale Graphene Industries specializes in the manufacture and functionalization of graphene and other nanomaterials for a variety of applications, including energy storage, electronics, and composites.
- Graphenea: Based in Spain, Graphenea is a leading producer of high-quality graphene and graphene oxide materials for a range of applications, including sensors, electronics, and energy storage.
- XG Sciences: Based in the USA, XG Sciences produces graphene and other advanced materials for use in energy storage, electronics, and composites.
- Nanotek Instruments: Based in the USA, Nanotek Instruments specializes in the production of graphene and other nanomaterials for a variety of applications, including energy storage, electronics, and biomedicine.
- G6 Materials: Based in the USA, G6 Materials produces high-quality graphene and graphene oxide materials for use in electronics, energy storage, and composites.
- Advanced Graphene Products: Based in the USA, Advanced Graphene Products specializes in the production of graphene and other advanced materials for a range of applications, including energy storage, composites, and coatings.
- Thomas Swan & Co.: Based in the UK, Thomas Swan & Co. is a leading producer of high-quality graphene and other advanced materials for use in electronics, energy storage, and composites.
- Group NanoXplore: Based in Canada, Group NanoXplore is a leading producer of graphene and graphene oxide materials for a range of applications, including energy storage, electronics, and composites.
- ACS Material: Based in the USA, ACS Material produces high-quality graphene and graphene oxide materials for use in electronics, energy storage, and composites.
- Angstron Materials: Based in the USA, Angstron Materials is a leading producer of high-quality graphene and graphene oxide materials for a range of applications, including electronics, energy storage, and composites.
It is worth noting that these are just a few of the companies that are active in the graphene production market, and there are many others that are also making significant contributions to the industry. Additionally, the graphene production market is still evolving, and it is likely that new companies will emerge as the industry continues to grow and mature.