Introduction
Faraday’s Laws of Electrolysis are fundamental principles in electrochemistry that describe the relationship between the amount of substance produced or consumed during electrolysis and the electrical charge passed through the electrolyte. These laws were formulated by the English scientist Michael Faraday in the 19th century and have since become crucial in understanding and manipulating chemical reactions involving electrolysis. In this article, we will delve into the details of Faraday’s laws and explore their diverse applications. Electrolysis is a process that uses an electric current to drive a non-spontaneous chemical reaction. It involves the decomposition of an electrolyte, typically a liquid or molten ionic compound, into its constituent elements or ions. Faraday’s Laws of Electrolysis provide quantitative relationships between the amount of substance transformed and the electrical charge passed through the electrolyte.
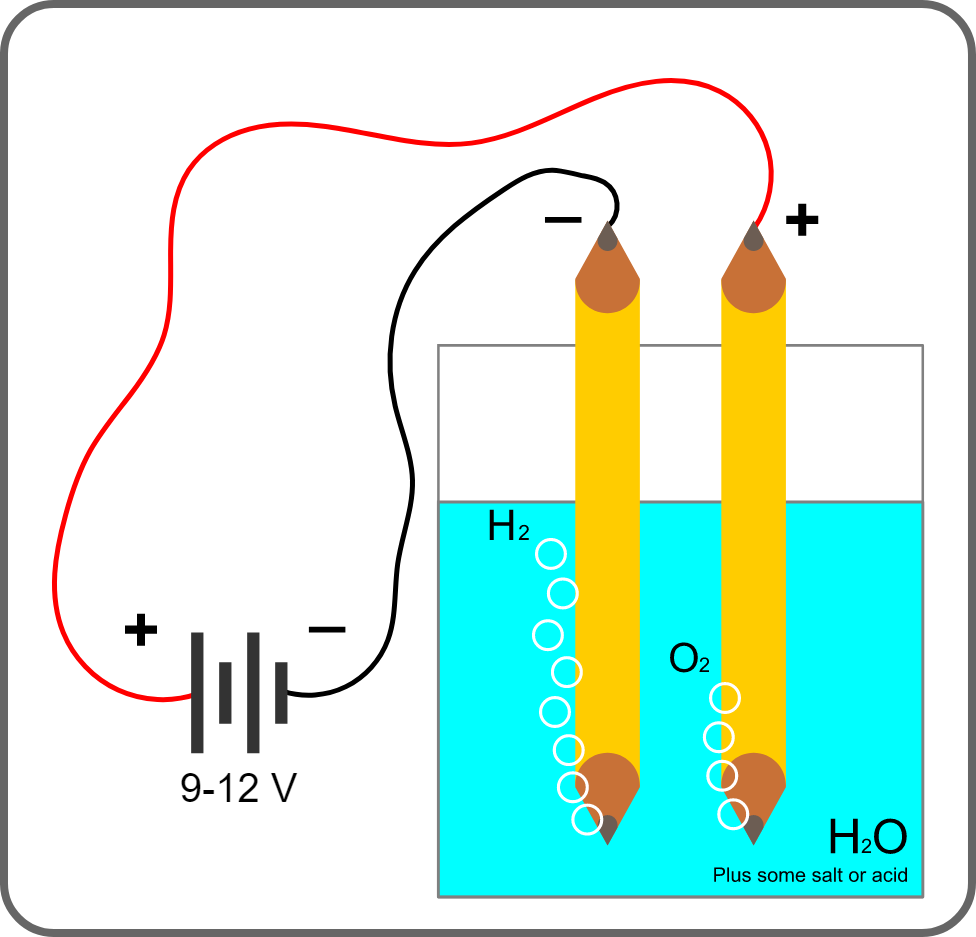
Faraday’s First Law of Electrolysis
Faraday’s First Law states that the amount of substance produced or consumed during electrolysis is directly proportional to the quantity of electricity passed through the electrolyte. In simpler terms, it means that the more electrical charge that flows through the electrolyte, the greater the amount of substance that is transformed.
Explanation of the Law
According to Faraday’s First Law, when an electric current is passed through an electrolyte, the number of ions involved in the chemical reaction is directly proportional to the quantity of electricity passed. This law is based on the concept that one mole of electrons carries a charge of 96,485 coulombs, which is known as the Faraday constant (F). In addition, Faraday’s First Law of Electrolysis states that ”the amount of substance produced or consumed during electrolysis is directly proportional to the quantity of electricity passed through the electrolyte”.
To understand this law, let’s consider an electrolytic cell where an electric current is passed through an electrolyte. The electrolyte contains ions that can move freely. When the current flows, it causes a chemical reaction at the electrodes, leading to the transformation of ions into their respective elements or compounds.
According to Faraday’s First Law, the extent of this transformation is determined by the quantity of electricity passed through the electrolyte. In simpler terms, the more electrical charge that flows through the electrolyte, the greater the amount of substance that is transformed.
This law is based on the concept that one mole of electrons carries a specific charge, known as the Faraday constant. The Faraday constant is approximately 96,485 coulombs per mole. Therefore, if a certain quantity of electricity is passed through the electrolyte, the number of moles of electrons involved in the chemical reaction can be determined.
By applying Faraday’s First Law, scientists and researchers can accurately calculate the amount of substance produced or consumed during electrolysis. This is essential for various applications, including chemical analysis, industrial processes, and research studies.
For instance, in electroplating, Faraday’s First Law helps in determining the amount of metal deposited on an electrode. This knowledge allows for precise control over the thickness and quality of the plated layer. Similarly, in electrolysis-based processes, such as the production of chemicals or the extraction of metals, Faraday’s First Law helps in measuring and controlling the number of substances involved.
In summary, Faraday’s First Law of Electrolysis establishes a direct relationship between the amount of substance transformed and the quantity of electricity passed through the electrolyte during electrolysis. This law plays a vital role in understanding and controlling chemical reactions in electrolytic cells, enabling various practical applications in different fields.
Formula and Units
The mathematical representation of Faraday’s First Law can be expressed using the formula:
Substance transformed (in moles)=Faraday constant(F)/Electric charge(in coulombs)
The SI unit for electric charge is the coulomb (C), and the Faraday constant is approximately 96,485 C/mol.
Application and Significance
Faraday’s First Law finds wide-ranging applications in various fields. It is used to determine the quantity of substances produced or consumed during electrolysis, which is crucial for chemical analysis, industrial processes, and research. For example, in electroplating, Faraday’s First Law helps calculate the amount of metal deposited on an electrode, ensuring precise control over the thickness and quality of the plated layer.
The application and significance of Faraday’s First Law of Electrolysis are extensive and crucial in various fields. Let’s explore how this law is applied and its significance in practical contexts:
- Electroplating: Electroplating is a widely used application of Faraday’s First Law. It involves the deposition of a thin layer of metal onto a surface by electrolysis. By controlling the amount of electricity passed through the electrolyte, the quantity of metal deposited can be precisely determined. This allows for the production of uniformly plated surfaces with desired thickness and quality. Electroplating finds applications in industries such as automotive, electronics, jewelry, and decorative coatings.
- Chemical Analysis: Faraday’s First Law plays a crucial role in quantitative chemical analysis. It helps determine the amount of substance present in a sample by measuring the quantity of electricity required for its electrolysis. This information is valuable in fields like forensic science, environmental analysis, and pharmaceutical research.
- Industrial Processes: Faraday’s First Law finds applications in various industrial processes that involve electrolysis. For example, the production of chlorine and sodium hydroxide through the electrolysis of brine relies on precise control over the amount of electricity passed to ensure efficient and economical production. Additionally, electrolysis is used in the extraction of metals and the synthesis of important chemicals, where Faraday’s First Law aids in determining the quantities of substances produced.
- Research and Development: In scientific research and development, Faraday’s First Law is essential for understanding and manipulating electrochemical reactions. It provides a quantitative basis for studying electrolysis and its impact on chemical transformations. Researchers can use this law to optimize electrolysis conditions, design electrochemical cells, and explore new applications in fields such as energy storage, materials science, and environmental remediation.
- Education and Fundamental Knowledge: Faraday’s First Law is a fundamental principle in electrochemistry and serves as the foundation for understanding electrolysis. It is taught in chemistry courses and textbooks to introduce students to the concept of electrochemical reactions and their quantitative aspects. Understanding Faraday’s First Law is essential for students pursuing fields related to chemistry, materials science, and engineering.
The significance of Faraday’s First Law lies in its ability to provide quantitative relationships between electricity, chemical reactions, and substances involved in electrolysis. This law enables precise control, measurement, and prediction of electrolysis processes, facilitating advancements in technology, industrial processes, scientific research, and innovation. Overall, Faraday’s First Law of Electrolysis has profound practical implications across various sectors, contributing to advancements in fields such as electroplating, chemical analysis, industrial processes, research, and education.
Faraday’s Second Law of Electrolysis
Faraday’s Second Law states that the masses of different substances transformed by the same amount of electricity are proportional to their respective chemical equivalent weights. This law allows for the calculation of the masses of substances involved in electrolysis.
Explanation of the Law
Faraday’s Second Law is based on the principle that during electrolysis, the amount of electricity required to transform a given number of ions into their respective elements or compounds is proportional to the chemical equivalent weight of those substances. The chemical equivalent weight represents the mass of a substance that reacts with one mole of electrons.
Faraday’s Second Law of Electrolysis states that the masses of different substances transformed by the same amount of electricity during electrolysis are proportional to their respective chemical equivalent weights. To understand this law, let’s consider an electrolytic cell where an electric current is passed through an electrolyte. The electrolyte contains different types of ions that can move freely. When the current flows, it causes chemical reactions at the electrodes, leading to the transformation of ions into their respective elements or compounds.
Faraday’s Second Law focuses on comparing the masses of substances involved in these chemical reactions. According to this law, when the same quantity of electricity passes through the electrolyte, the masses of the substances transformed are directly proportional to their chemical equivalent weights. The chemical equivalent weight represents the mass of a substance that reacts with one mole of electrons. It is calculated based on the substance’s molar mass and the number of electrons involved in the chemical reaction. The chemical equivalent weight allows for comparing the masses of different substances in terms of their reactivity with the same quantity of electricity.
By applying Faraday’s Second Law, scientists and researchers can determine the mass of a substance transformed during electrolysis when the quantity of electricity passing through the electrolyte is known. This law provides a basis for calculating the masses of substances involved in various electrolysis processes.
The significance of Faraday’s Second Law lies in its practical applications, particularly in electrochemical processes. It enables precise control over the amount of material deposited or consumed during electrolysis, contributing to efficient electroplating, electrorefining, and other electrochemical processes.
For example, in electroplating, where a metal is deposited onto a surface, Faraday’s Second Law helps in determining the mass of the metal deposited based on the quantity of electricity passed. This allows for precise control over the thickness and quality of the plated layer.
Similarly, in electrorefining, where impure metals are purified through electrolysis, Faraday’s Second Law aids in calculating the mass of impurities that can be removed based on the applied quantity of electricity. This ensures the production of high-purity refined metals.
In summary, Faraday’s Second Law of Electrolysis establishes a relationship between the masses of substances transformed and their respective chemical equivalent weights when the same amount of electricity passes through the electrolyte. This law is essential in calculating the masses of substances involved in electrolysis processes, enabling precise control and understanding of electrochemical reactions in various practical applications.
Formula and Units
The mathematical representation of Faraday’s Second Law can be expressed using the formula:
Mass of substance transformed=Equivalent weight×Faraday constant Electric charge
The mass of the substance transformed is typically measured in grams (g), and the equivalent weight is expressed in grams per mole (g/mol).
Application and Significance
Faraday’s Second Law is widely used in electrochemical processes and provides crucial information for industrial applications. It enables precise control over the amount of material deposited or consumed during electrolysis, allowing for efficient electroplating, electrorefining, and other electrochemical processes.
Uses of Faraday’s Laws of Electrolysis
Faraday’s Laws of Electrolysis have numerous practical applications. Here are some notable uses:
Electroplating
Electroplating is a process that involves depositing a layer of metal onto a surface by electrolysis. Faraday’s laws are instrumental in determining the amount of metal deposited during electroplating. This enables precise control over the plated layer’s thickness, adhesion, and quality. Electroplating is widely used in various industries, including automotive, jewelry, electronics, and decorative coatings. Certainly! Faraday’s Laws of Electrolysis are fundamental principles that govern the process of electroplating. Electroplating involves depositing a layer of metal onto a conductive surface through the use of an electrolytic cell. Here’s a brief explanation of Faraday’s Laws of Electrolysis as they apply to electroplating:
- Faraday’s First Law: This law states that the amount of chemical reaction occurring at an electrode is directly proportional to the quantity of electricity passed through the electrolyte. In electroplating, it means that the mass of the metal deposited on the cathode (the object being plated) is directly proportional to the amount of electric charge (current) passed through the electrolyte. The equation representing this relationship is:Mass of substance deposited = (Current × Time) / (Faraday’s constant × Equivalent weight)
- Faraday’s Second Law: This law states that the ratio of the masses of different elements being deposited during electrolysis equals the percentage of their respective chemical equivalent weights. In electroplating, this law implies that their equivalent weights determine the ratio of the masses of different metals deposited on the cathode. The equation representing this relationship is: Mass of metal A / Mass of metal B = Equivalent weight of metal A / Equivalent weight of metal B
These laws provide a quantitative understanding of the electroplating process and are essential for controlling and predicting the deposition of metals during electroplating.
It’s worth noting that the laws assume ideal conditions, such as 100% current efficiency and no side reactions. In practice, factors like solution composition, temperature, and electrode material can influence the actual plating process.
If you’re interested in learning more about electroplating and Faraday’s Laws of Electrolysis in detail, you can refer to textbooks on electrochemistry or specifically on electroplating, as mentioned in the previous response. These resources delve deeper into the principles, techniques, and applications of electroplating.
Electrorefining
Electrorefining is a method used to purify metals through electrolysis. Faraday’s laws help in calculating the amount of impurities that can be removed from the metal. Electrorefining is commonly employed in the extraction and purification of copper, zinc, and other metals, ensuring high purity and desired properties. Certainly! Faraday’s Laws of Electrolysis also play a crucial role in the process of electrorefining. Electrorefining is a technique used to purify metals through electrolysis, particularly copper, zinc, nickel, and lead. Let’s explore how Faraday’s Laws apply to electrorefining:
- Faraday’s First Law: As mentioned earlier, this law states that the amount of chemical reaction at an electrode is directly proportional to the quantity of electricity passed through the electrolyte. In the context of electrorefining, it means that the mass of metal deposited on the cathode is directly related to the amount of electric charge passed through the electrolyte.
During electrorefining, impure metal is used as the anode, and a pure metal sheet serves as the cathode. As an electric current is applied, metal ions from the anode dissolve into the electrolyte, while pure metal ions are reduced and deposited onto the cathode. According to Faraday’s First Law, the mass of pure metal deposited at the cathode is directly proportional to the amount of electric charge (current) passing through the cell.
- Faraday’s Second Law: This law states that the ratio of masses of different elements being deposited during electrolysis is equal to the ratio of their respective chemical equivalent weights. In the case of electrorefining, this law implies that the ratio of the masses of different impurities deposited on the cathode is determined by their respective chemical equivalent weights.
During the electrorefining process, impurities present in the anode dissolve into the electrolyte and may also get deposited on the cathode. The ratio of the masses of these impurities that deposit on the cathode is determined by their equivalent weights, as per Faraday’s Second Law.
Faraday’s Laws of Electrolysis are essential for controlling and optimizing the electrorefining process, as they help determine the efficiency of metal purification and provide a quantitative understanding of the deposition of impurities and the desired metal onto the cathode.
To explore electrorefining and Faraday’s Laws in greater detail, you can refer to textbooks on electrochemistry or metallurgy that cover the topic of electrorefining. These resources will provide comprehensive information on the principles, techniques, and applications of electrorefining for various metals.
Electrolysis in Industry
Faraday’s laws play a crucial role in industrial electrolysis processes. They help determine the amount of substance produced or consumed during the electrolysis of various compounds. Industries utilize electrolysis for the production of chemicals, such as chlorine and sodium hydroxide, as well as for the extraction of metals and the synthesis of important compounds. Faraday’s Laws of Electrolysis are of significant importance in the electrolysis industry. They provide fundamental principles that govern the process of electrolysis and play a crucial role in various industrial applications. Let’s explore how Faraday’s Laws apply to the electrolysis industry:
- Faraday’s First Law: This law states that the amount of chemical reaction occurring at an electrode is directly proportional to the quantity of electricity passed through the electrolyte. In the electrolysis industry, Faraday’s First Law is used to determine the amount of material produced or consumed during electrolysis.
For example, in the production of metals like aluminum, sodium, and magnesium through electrolysis, Faraday’s First Law helps determine the amount of metal that can be obtained based on the quantity of electricity passed through the electrolytic cell. It is used to calculate the production rate, optimize process conditions, and ensure efficient resource utilization.
- Faraday’s Second Law: This law states that the ratio of masses of different elements being deposited during electrolysis is equal to the percentage of their respective chemical equivalent weights. In the electrolysis industry, Faraday’s Second Law is employed to control the composition and purity of products.
For instance, in the production of chlorine and sodium hydroxide through the electrolysis of brine (salt water) in a chloralkali process, Faraday’s Second Law helps ensure the correct ratio of chlorine and sodium hydroxide production. By considering the equivalent weights of chlorine and sodium, the desired product ratio can be achieved, leading to efficient and balanced production.
Faraday’s Laws of Electrolysis provide a quantitative understanding of the electrochemical reactions taking place in various electrolysis processes. They are essential for process control, optimizing energy consumption, determining production rates, and ensuring the quality and purity of electrolysis products in industries such as metallurgy, chemical manufacturing, and electrochemical synthesis.
To delve deeper into the application of Faraday’s Laws in the electrolysis industry, you can refer to textbooks and resources on electrochemistry, industrial electrolysis, and specific industrial processes that utilize electrolysis. These sources will provide more detailed insights into the practical implementation of Faraday’s Laws in industrial electrolysis applications.
Electrolysis in Medicine
Electrolysis has applications in medicine, particularly in the field of dermatology. It is commonly used for the removal of unwanted hair through a process called electrolysis hair removal. Faraday’s laws aid in controlling the amount of electrolysis applied, ensuring safe and effective hair removal. Faraday’s Laws of Electrolysis also find applications in the field of medicine, particularly in electrolysis-based medical procedures. Here’s how Faraday’s Laws apply to electrolysis in medicine:
- Electrolysis for Hair Removal: Electrolysis is a common method used for permanent hair removal. It involves the insertion of a fine needle-like electrode into individual hair follicles. Electrical current is then applied to the electrode, leading to chemical reactions at the follicle that ultimately destroy the hair growth cells.
Faraday’s First Law is relevant in electrolysis for hair removal as it governs the amount of electricity required to produce the desired chemical reaction. The quantity of electricity delivered through the electrolysis process determines the efficiency and effectiveness of hair removal. Operators must ensure that the appropriate amount of current is applied to each hair follicle to achieve optimal results.
- Electrolysis for Skin Lesions and Blemishes: Electrolysis is also employed in medical settings for the treatment of certain skin lesions and blemishes, such as skin tags, moles, and warts. In this context, electrolysis is used to selectively destroy the affected tissue while minimizing damage to surrounding healthy skin.
Faraday’s First Law guides the application of electrical current in electrolysis-based treatments for skin lesions. By controlling the quantity of electricity delivered, medical professionals can ensure the targeted destruction of the lesion while avoiding excessive damage to the surrounding tissue.
Faraday’s Laws of Electrolysis provide a scientific basis for the precise application of electrical current in medical electrolysis procedures. By understanding these laws, healthcare practitioners can optimize treatment outcomes, minimize patient discomfort, and ensure the safety and effectiveness of electrolysis-based medical interventions.
It’s important to note that electrolysis procedures in medicine should be performed by trained professionals with appropriate expertise and adherence to established protocols and safety guidelines.
Top 10 books on Faraday Laws of Electrolysis
Here are some recommended books that extensively cover Faraday’s Laws of Electrolysis along with other relevant topics in electrochemistry:
- “Electrochemical Methods: Fundamentals and Applications” by Allen J. Bard and Larry R. Faulkner
- “Electrochemistry” by Philip N. Ross
- “Electrochemical Systems” by John Newman and Karen E. Thomas-Alyea
- “Electrochemical Methods in Archaeometry, Conservation and Restoration” edited by Orsolya Török and Kálmán B. Gyarfás
- “Electrochemical Techniques in Corrosion Science and Engineering” by Robert G. Kelly and John R. Scully
- “Physical Electrochemistry: Fundamentals, Techniques, and Applications” by Eliezer Gileadi
- “Electrochemistry for Chemists” by Tadeusz Michałowski
- “Electrochemistry: Principles, Methods, and Applications” by Christopher M. A. Brett and Ana Maria Oliveira Brett
- “Modern Electrochemistry 1: Ionics” by John O’M. Bockris, Amulya K. N. Reddy, and Maria E. Gamboa-Aldeco
- “Modern Electrochemistry 2A: Fundamentals of Electrodics” by John O’M. Bockris, Amulya K. N. Reddy, and Maria E. Gamboa-Aldeco
These books provide a solid foundation in electrochemistry, including the principles and applications of Faraday’s Laws of Electrolysis. They cover a wide range of topics, from the fundamentals to more specialized areas within the field.
Conclusion
Faraday’s Laws of Electrolysis, formulated by Michael Faraday, provide essential insights into the relationship between electricity and chemical reactions during electrolysis. These laws have found extensive applications in various fields, including electroplating, electrorefining, industrial processes, and medicine. Understanding Faraday’s laws enables precise control over electrolysis processes and contributes to advancements in technology, scientific research, and industrial production.
FAQs
1. What is Faraday’s First Law of Electrolysis?
Faraday’s First Law of Electrolysis states that the amount of substance produced or consumed during electrolysis is directly proportional to the quantity of electricity passed through the electrolyte.
2. How do Faraday’s Laws of Electrolysis contribute to electroplating?
Faraday’s Laws of Electrolysis help calculate the amount of metal deposited during electroplating, ensuring precise control over the thickness and quality of the plated layer.
3. What are some applications of Faraday’s Laws of Electrolysis in medicine?
Faraday’s Laws find applications in electrolysis-based procedures, such as electrolysis hair removal, commonly used in dermatology for the removal of unwanted hair.
4. Can you explain the concept of electrorefining?
Electrorefining is a process that uses electrolysis to purify metals. Faraday’s Laws of Electrolysis are instrumental in calculating the amount of impurities that can be removed from the metal, resulting in high-purity refined metals.
5. How do Faraday’s Laws of Electrolysis help in the purification of metals?
Faraday’s Second Law of Electrolysis allows for the calculation of the masses of substances involved in electrolysis. This enables precise control over the amount of impurities that can be removed during electrorefining, contributing to the purification of metals.
