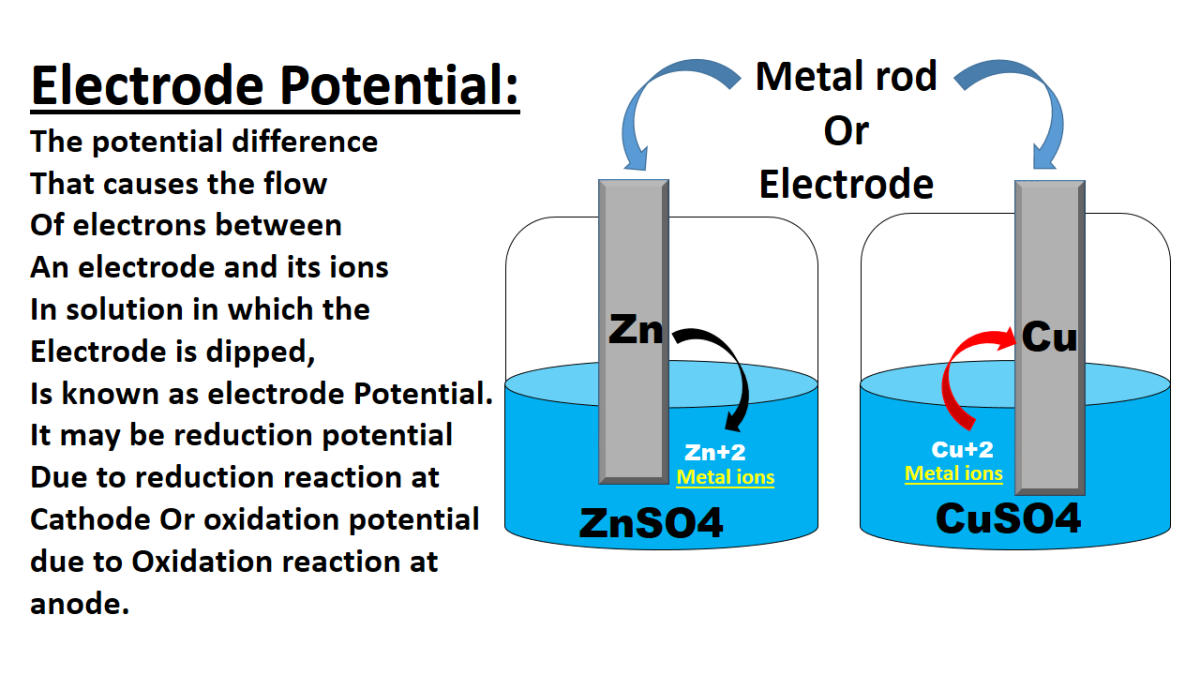
Introduction
Electrode potential, also known as electrode potential difference or electrode potential difference, is a measure of the tendency of an electrode to gain or lose electrons in an electrochemical cell. It is a fundamental concept in electrochemistry and plays a crucial role in understanding and predicting the behavior of electrodes in various applications. Here is an explanation of electrode potential and its applications.
Definition of Electrode Potential
Electrode potential is defined as the electric potential difference between an electrode and the surrounding electrolyte solution when no current is flowing through the cell. It represents the tendency of an electrode to undergo a reduction or oxidation reaction. Electrode potential is measured against a reference electrode, typically a standard hydrogen electrode (SHE) or a saturated calomel electrode (SCE).
- Standard Electrode Potential: The standard electrode potential (E°) is the electrode potential measured under standard conditions, which include a concentration of 1 M for all ions and a temperature of 25°C. The standard electrode potential values are tabulated and used as a reference for comparing the tendencies of different electrodes to undergo reduction or oxidation reactions.
- Nernst Equation: The Nernst equation relates the electrode potential to the concentration of species involved in the redox reaction. It is given by E = E° – (RT/nF) * ln(Q), where E is the electrode potential, E° is the standard electrode potential, R is the gas constant, T is the temperature, n is the number of electrons transferred in the redox reaction, F is Faraday’s constant, and Q is the reaction quotient.
Electrode potential, also known as electrode potential difference or electrode potential, is a fundamental concept in electrochemistry that plays a crucial role in understanding the behavior of electrodes in electrochemical cells. It refers to the electric potential difference between an electrode and the surrounding electrolyte solution when no current is flowing through the cell. Electrode potential is a measure of the tendency of an electrode to gain or lose electrons and undergo reduction or oxidation reactions. In this article, we will explore electrode potentials in detail, including their definition, factors affecting them, measurement methods, and their significance in various electrochemical applications.
Standard Electrode Potential: The standard electrode potential (E°) is the electrode potential measured under standard conditions, including a concentration of 1 M for all ions and a temperature of 25°C. Standard electrode potentials are tabulated and serve as reference values for comparing the tendencies of different electrodes to undergo reduction or oxidation reactions. The standard hydrogen electrode is assigned an arbitrary potential of 0 volts, and other electrode potentials are expressed relative to it.
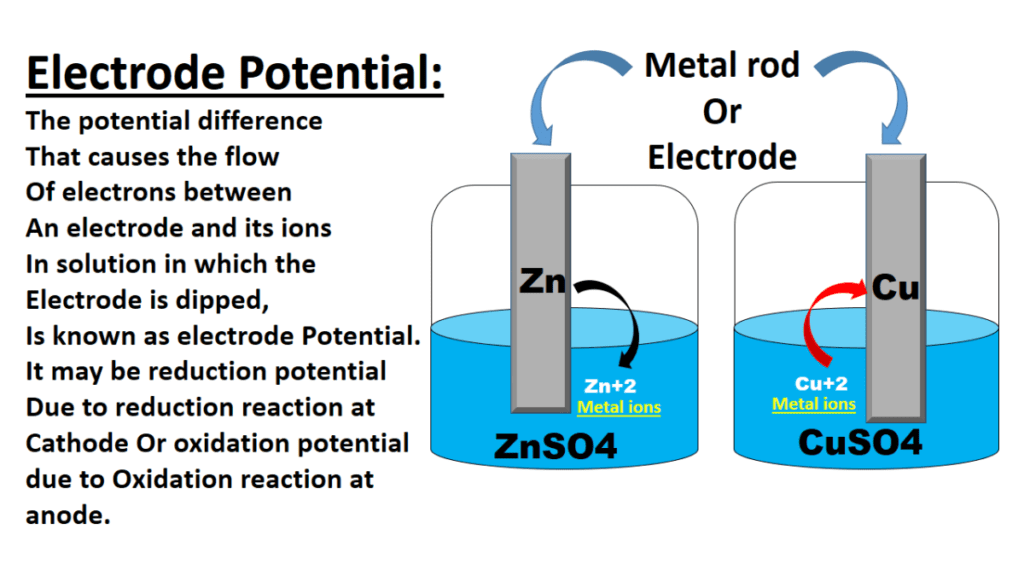
Factors Affecting Electrode Potential
Several factors influence the electrode potential of a given electrode:
- Nature of the Electrode Material: The intrinsic properties of the electrode material, such as its electronic structure, crystal structure, and surface morphology, can significantly affect its electrode potential. Different materials exhibit different tendencies to undergo oxidation or reduction reactions.
- The concentration of Species in Solution: The concentration of reactants and products in the electrolyte solution can influence the electrode potential. According to the Nernst equation, the electrode potential depends logarithmically on the concentrations of species involved in the redox reaction.
- Temperature: The temperature affects the electrode potential, primarily through its impact on the reaction rates of the redox reactions. Higher temperatures can increase the kinetics of the reaction, leading to changes in the electrode potential.
Measurement Methods
There are various methods used to measure electrode potentials:
- Potentiometry: Potentiometry is a technique that measures the voltage or potential difference between an electrode and a reference electrode. It allows the determination of the electrode potential of an electrode immersed in an electrolyte solution. Potentiometric measurements are commonly performed using a high-impedance voltmeter or a potentiostat.
- Galvanic Cell: Galvanic cells, also known as voltaic cells, can be used to indirectly measure electrode potentials. In a galvanic cell, the potential difference generated by the redox reaction at the electrode is measured as electrical energy. By comparing the potential difference of the cell with a reference electrode, the electrode potential can be determined.
Applications of Electrode Potential
Corrosion Prevention
Electrode potential plays a vital role in understanding and preventing corrosion. By controlling the electrode potentials of metals, it is possible to inhibit or minimize the oxidation (corrosion) of metal surfaces. Protective coatings, sacrificial anodes, and impressed current systems are employed to control the electrode potential and protect metals from corrosion.
Electrode potential plays a crucial role in corrosion protection strategies. Corrosion is an electrochemical process where metals react with their environment, leading to the deterioration of the metal surface. By understanding and controlling the electrode potentials of metal surfaces, it is possible to minimize or prevent corrosion. Here is an explanation of how electrode potential is involved in corrosion protection:
- Galvanic Corrosion: Galvanic corrosion occurs when two dissimilar metals are in contact with each other in the presence of an electrolyte. The metals have different electrode potentials, and an electrochemical cell is formed. One metal acts as the anode and undergoes oxidation (corrosion), while the other metal acts as the cathode and undergoes reduction. The potential difference between the two metals drives the corrosion process. By selecting metals with similar electrode potentials, the potential difference and the rate of galvanic corrosion can be minimized.
- Sacrificial Anodes: Sacrificial anodes are commonly used in corrosion protection systems. These are metals with more negative electrode potentials than the metal being protected. The sacrificial anode is connected to the metal structure that needs protection, and it corrodes preferentially, sacrificing itself to protect the primary metal. The more negative electrode potential of the sacrificial anode ensures that it undergoes oxidation (corrosion) instead of the protected metal.
- Cathodic Protection: Cathodic protection is a technique used to protect metal structures by making them the cathode in an electrochemical cell. It involves the application of a direct current (DC) to the metal surface, shifting its electrode potential towards more negative values. This negative potential prevents the metal from undergoing oxidation and effectively protects it from corrosion. Cathodic protection can be achieved through impressed current systems or by using sacrificial anodes.
- Coatings and Inhibitors: Coatings and inhibitors are widely used for corrosion protection. These materials provide a barrier between the metal surface and the corrosive environment or interfere with the electrochemical reactions occurring at the surface. The choice of coatings and inhibitors is based on their ability to shift the electrode potential of the metal towards more positive values, reducing its tendency to undergo oxidation. By modifying the electrode potential, coatings, and inhibitors can effectively protect metals from corrosion.
- Monitoring and Control: Electrode potential measurements are employed to monitor and control the corrosion protection systems. By monitoring the electrode potentials of metal structures, it is possible to assess the effectiveness of corrosion protection measures and detect any changes or deviations. Adjustments can then be made to optimize the protection system and ensure that the electrode potential remains within the desired range for corrosion prevention.
Overall, the electrode potential is a critical factor in corrosion protection strategies. By selecting metals with similar electrode potentials, using sacrificial anodes, applying for cathodic protection, utilizing coatings and inhibitors, and monitoring the electrode potentials of metal structures, it is possible to effectively protect metals from the damaging effects of corrosion. Understanding and controlling electrode potentials are essential in designing and implementing corrosion protection measures for various industries, including transportation, infrastructure, marine, and oil and gas.
Batteries and Fuel Cells
Electrode potential is crucial in the operation of batteries and fuel cells, which rely on redox reactions for energy storage and conversion. The electrode potential difference between the anode and cathode determines the cell voltage and the overall energy output of the device.
Electrode potential plays a fundamental role in the operation of batteries and fuel cells, which are electrochemical devices that convert chemical energy into electrical energy. The electrode potentials of the anode and cathode materials determine the cell voltage and overall performance of these devices. Here’s how electrode potential is involved in batteries and fuel cells:
- Batteries: Batteries are electrochemical devices that store and release electrical energy through redox reactions. They consist of one or more cells connected in series or parallel. Each cell contains two electrodes (anode and cathode) and an electrolyte.
Anode: The anode is the electrode where oxidation occurs. It releases electrons to the external circuit and undergoes oxidation, generating cations (positive ions) in the electrolyte. The electrode potential of the anode material determines the voltage at which the oxidation reaction takes place.
Cathode: The cathode is the electrode where reduction occurs. It accepts electrons from the external circuit and undergoes reduction, consuming cations from the electrolyte. The electrode potential of the cathode material determines the voltage at which the reduction reaction takes place.
Cell Voltage: The difference in electrode potentials between the anode and cathode materials determines the cell voltage of the battery. The cell voltage is the potential difference or electromotive force (EMF) produced by the redox reactions occurring at the electrodes. It represents the maximum electrical potential difference available from the battery.
Fuel Cells: Fuel cells are electrochemical devices that directly convert the chemical energy of a fuel (such as hydrogen, methanol, or hydrocarbons) and an oxidant (such as oxygen or air) into electrical energy. They operate continuously as long as the fuel and oxidant are supplied.
Anode: The anode in a fuel cell is where the fuel is oxidized. It releases electrons to the external circuit and generates cations (positive ions) in the electrolyte. The electrode potential of the anode material determines the voltage at which the oxidation reaction takes place.
Cathode: The cathode in a fuel cell is where the oxidant (usually oxygen) is reduced. It accepts electrons from the external circuit and consumes cations from the electrolyte. The electrode potential of the cathode material determines the voltage at which the reduction reaction takes place.
Cell Voltage: The difference in electrode potentials between the anode and cathode materials determines the cell voltage of the fuel cell. The cell voltage represents the maximum electrical potential difference available from the fuel cell.
In both batteries and fuel cells, the electrode potentials of the anode and cathode materials determine the thermodynamics and kinetics of the redox reactions. The driving force for the electrochemical reactions is the difference in electrode potentials, which is related to the free energy change (ΔG) of the redox reactions. The higher the difference in electrode potentials, the greater the cell voltage and the more energy can be extracted from the device.
It is important to note that the electrode potentials can be influenced by various factors, including the nature of the electrode materials, the concentration of species in the electrolyte, temperature, and other external conditions. Designing and selecting electrode materials with appropriate electrode potentials is crucial for optimizing the performance and efficiency of batteries and fuel cells.
Furthermore, in fuel cells, the electrode potentials are also influenced by the fuel and oxidant supply rates, reactant concentrations, and the reaction kinetics at the electrode surfaces. Proper management and control of these factors are essential for maintaining efficient fuel cell operation.
Overall, electrode potential plays a central role in determining the cell voltage and overall performance of batteries and fuel cells. Understanding and controlling the electrode potentials of the anode and cathode materials are crucial for optimizing the energy conversion efficiency and extending the operating life.
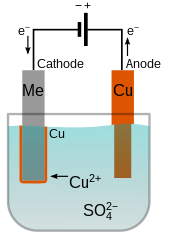
Electroplating
Electroplating is a process that utilizes electrode potential to deposit a metal coating onto a substrate. By controlling the electrode potential, it is possible to selectively plate a metal onto a surface, providing protection, decoration, or enhanced properties.
Electrode potential plays a critical role in the process of electroplating, which is a widely used technique for depositing a layer of metal onto the surface of another metal object. Electroplating involves the use of an electrolytic cell where the object to be plated serves as the cathode, and a metal electrode serves as the anode. The electrode potentials of the anode and cathode materials are crucial in determining the plating process and the quality of the plated layer. Here’s how electrode potential is involved in electroplating:
- Anode: The anode in an electroplating cell is typically made of the metal that will be deposited onto the object. For example, if gold plating is desired, the anode will be made of gold. The anode material undergoes oxidation and dissolves into the electrolyte solution, supplying metal cations (positive ions) for the plating process. The electrode potential of the anode material determines the rate of dissolution and the concentration of metal cations in the electrolyte.
- Cathode: The object to be plated serves as the cathode in the electroplating cell. It attracts metal cations from the electrolyte and facilitates their reduction onto its surface, forming a layer of the desired metal. The electrode potential of the cathode material influences the rate at which the metal cations are reduced and determines the quality and thickness of the plated layer.
- Electrolyte Solution: The electrolyte solution in the electroplating cell contains metal ions corresponding to the desired plated metal. These metal ions come from the anode as it undergoes oxidation. The concentration of metal ions in the electrolyte solution is influenced by the electrode potentials of the anode and cathode materials. Higher electrode potentials at the anode favor a higher concentration of metal ions in the electrolyte, leading to a faster and more efficient plating process.
- Cell Voltage: The cell voltage of the electroplating cell is determined by the difference in electrode potentials between the anode and cathode materials. The cell voltage provides the driving force for the redox reaction, where metal cations are reduced at the cathode and the anode undergoes oxidation. The magnitude of the cell voltage affects the plating rate and the quality of the plated layer. Controlling the cell voltage is crucial for achieving the desired plating thickness and uniformity.
- Faraday’s Law: Faraday’s law of electrolysis relates the amount of metal deposited during electroplating to the current passing through the cell and the electrochemical equivalent of the metal. The electrode potential influences the current flow and, therefore, the rate of metal deposition. The electrochemical equivalent is a constant that relates the amount of metal deposited to the charge passing through the cell. By controlling the electrode potential and the current, the thickness and quality of the plated layer can be precisely controlled.
Controlling the electrode potentials and other parameters in electroplating is essential for achieving successful and high-quality plating results. Factors such as the composition and concentration of the electrolyte, temperature, current density, and plating time also play a role in determining the final plated layer’s properties. By carefully selecting the anode and cathode materials, optimizing the electrode potentials, and controlling the plating parameters, electroplating processes can be tailored to meet specific requirements, such as thickness, adhesion, corrosion resistance, and aesthetics. Electroplating finds extensive applications in industries such as automotive, electronics, jewelry, and decorative coatings.
pH Measurement
Electrode potential is used in pH measurement through the use of pH electrodes. The potential difference between a glass electrode and a reference electrode, such as a calomel electrode, changes with the pH of the solution. This change in potential allows for the determination of the pH value.
Electrode potential is closely associated with pH measurement, as it forms the basis for the operation of pH electrodes. pH is a measure of the acidity or alkalinity of a solution and is defined as the negative logarithm of the hydrogen ion concentration ([H+]). pH electrodes are electrochemical sensors that rely on the electrode potential to determine the pH of a solution. Here’s how electrode potential is involved in pH measurement:
- pH Electrode: A typical pH electrode consists of two electrodes immersed in an electrolyte solution. The first electrode is a glass electrode, which is selective to hydrogen ions. It consists of a glass membrane with a thin layer of hydrated metal ions. The second electrode is a reference electrode, usually a silver/silver chloride electrode or a calomel electrode. These electrodes work together to measure the pH of the solution.
- Glass Electrode: The glass electrode is the key component responsible for sensing the hydrogen ion concentration and providing a voltage signal. The glass membrane is made of a special glass composition that is sensitive to changes in [H+]. When the glass membrane comes into contact with the solution, hydrogen ions from the solution diffuse into the glass and replace the metal ions within the membrane, creating a potential difference. This potential difference is related to the hydrogen ion concentration and is converted into a pH value.
- Electrode Potential and pH: The electrode potential of the glass electrode is directly related to the pH of the solution. As the hydrogen ion concentration increases (lower pH), the electrode potential becomes more positive. Conversely, as the hydrogen ion concentration decreases (higher pH), the electrode potential becomes more negative. This change in electrode potential is the basis for determining the pH value.
- Calibration: To accurately measure pH, pH electrodes require calibration. Calibration involves immersing the electrode in solutions of known pH, typically pH 4, 7, and 10, and adjusting the electrode potential to match the expected values. The electrode potential is adjusted by adjusting the reference electrode and, if necessary, performing a slope adjustment. Calibration ensures the accuracy and reliability of the pH measurement.
- Nernst Equation: The Nernst equation is used to relate the electrode potential of the glass electrode to the hydrogen ion concentration and pH. The Nernst equation states that the electrode potential is proportional to the logarithm of the ratio of the hydrogen ion concentration in the solution to the hydrogen ion concentration in a standard solution. This mathematical relationship allows the conversion of electrode potential into pH values.
By measuring the electrode potential of the glass electrode and applying the Nernst equation, pH meters can accurately determine the pH of a solution. pH measurement finds extensive applications in various fields, including chemistry, biology, environmental monitoring, water treatment, food and beverage, and pharmaceuticals.
It is important to note that pH measurement is affected by factors such as temperature, ionic strength, electrode conditioning, and electrode aging. Regular calibration, proper maintenance, and careful handling of pH electrodes are necessary to ensure accurate and reliable pH measurements.
Sensors and Biosensors
Electrode potential is employed in the development of various sensors and biosensors. For example, electrochemical sensors utilize the change in electrode potential due to the interaction of analytes with specific electrodes to detect and quantify substances in various fields, including environmental monitoring, medical diagnostics, and food safety. Electrode potential plays a crucial role in the operation of sensors and biosensors, which are devices used to detect and measure various chemical and biological substances. These electrochemical devices rely on the electrode potential to facilitate the desired reactions and generate electrical signals proportional to the target analyte concentration. Here’s how electrode potential is involved in sensors and biosensors:
- Working Principle: Sensors and biosensors typically consist of an electrode or an array of electrodes that interact with the target analyte in the sample. The electrode potential is adjusted and optimized to enable the desired electrochemical reactions to occur. This potential difference drives the redox reactions, electron transfer, or ion exchange processes necessary for the detection and measurement of the analyte.
- Sensing Mechanisms: Sensors and biosensors utilize different sensing mechanisms to detect specific analytes. These mechanisms can include amperometry, potentiometry, voltammetry, impedance spectroscopy, and conductometry, among others. In each case, the electrode potential is adjusted to enable the specific electrochemical reaction that produces a measurable signal.
- Transduction: The electrochemical reactions occurring at the electrode surface generate an electrical signal that can be measured and quantified. The electrode potential affects the magnitude and nature of this signal, such as the current, voltage, or impedance changes, which are proportional to the concentration of the analyte being measured. The electrode potential optimization is crucial for achieving high sensitivity, selectivity, and accuracy of the sensor or biosensor.
- Surface Modification: In many cases, the electrode surface is modified or functionalized to enhance the sensitivity and selectivity of the sensor or biosensor. Surface modification can involve the immobilization of specific molecules, such as enzymes, antibodies, DNA, or other recognition elements, to selectively interact with the target analyte. The electrode potential influences the attachment, orientation, and stability of the functional molecules on the electrode surface, enabling efficient analyte recognition and detection.
- Calibration and Optimization: Similar to pH electrodes, sensors, and biosensors require calibration to ensure accurate and reliable measurements. Calibration involves determining the relationship between the electrode potential and the concentration of the target analyte by using calibration standards of known concentrations. Additionally, optimizing the electrode potential, along with other parameters such as scan rate, potential range, and reaction time, is crucial for achieving the desired sensitivity, dynamic range, and response time of the sensor or biosensor.
- Application Areas: Sensors and biosensors find extensive applications in various fields, including environmental monitoring, clinical diagnostics, food and beverage analysis, industrial process control, and biomedical research. They are used to detect and measure substances such as glucose, cholesterol, pH, ions, gases, biomarkers, pathogens, and toxins. The electrode potential optimization is tailored to the specific application and target analyte to achieve accurate and reliable measurements.
Overall, electrode potential plays a fundamental role in sensors and biosensors by enabling the desired electrochemical reactions, facilitating transduction, and generating electrical signals proportional to the target analyte concentration. The proper adjustment and optimization of the electrode potential, along with other parameters, are essential for achieving the desired sensitivity, selectivity, and accuracy in sensor and biosensor applications.
Corrosion Testing and Materials Characterization
Electrode potential measurements are used in corrosion testing to evaluate the corrosion resistance of materials and coatings. By monitoring the changes in electrode potential, the corrosion behavior and performance of materials can be assessed. Electrode potential plays a significant role in corrosion testing and materials characterization, as it provides valuable information about the corrosion behavior and properties of different materials. By measuring the electrode potential of a material in a corrosive environment, various aspects of corrosion can be evaluated, such as the corrosion rate, susceptibility to corrosion, and effectiveness of corrosion protection methods. Here’s how electrode potential is involved in corrosion testing and materials characterization:
- Corrosion Potential: The corrosion potential, also known as the open circuit potential, is the electrode potential of a metal or material in its passive state when it is exposed to a corrosive environment. It represents the thermodynamic tendency of the material to corrode or resist corrosion. By measuring the corrosion potential, information about the relative nobility or reactivity of the material can be obtained. A more negative corrosion potential indicates a higher tendency for corrosion, while a more positive potential indicates a higher resistance to corrosion.
- Potentiodynamic Polarization: Potentiodynamic polarization is a commonly used electrochemical technique in corrosion testing. It involves scanning the electrode potential of a material over a range of values while measuring the resulting current response. The polarization curve obtained from this technique provides information about the electrochemical reactions occurring on the material’s surface and its corrosion behavior. The polarization resistance, which is derived from the slope of the polarization curve at the corrosion potential, is a measure of the material’s resistance to corrosion.
- Galvanic Corrosion: Galvanic corrosion occurs when two dissimilar metals or materials are electrically connected in the presence of an electrolyte. The electrode potential of each material determines its nobility or reactivity, and the potential difference between them drives the galvanic corrosion process. By measuring the electrode potentials of the different materials in the galvanic couple, the likelihood and severity of galvanic corrosion can be assessed. Matching electrode potentials or minimizing the potential difference between materials helps prevent galvanic corrosion.
- Corrosion Rate Measurement: The electrode potential of a material can be used to estimate the corrosion rate. By monitoring changes in the electrode potential over time, it is possible to observe trends in corrosion behavior. A more negative shift in the electrode potential indicates a higher corrosion rate, while a stable or positive shift indicates a lower corrosion rate. By combining the electrode potential data with other corrosion parameters, such as the Tafel slope and corrosion current, more accurate corrosion rate calculations can be obtained.
- Materials Characterization: Electrode potential measurements are also used in materials characterization to understand the electrochemical behavior and properties of different materials. By comparing the electrode potentials of different alloys or coatings, the effectiveness of corrosion-resistant materials or protective coatings can be assessed. The electrode potential data can provide insights into the passivation behavior, pitting resistance, and overall corrosion performance of materials.
- Corrosion Testing Methods: Various corrosion testing methods, such as electrochemical impedance spectroscopy (EIS) and cyclic voltammetry, rely on electrode potential measurements to assess the corrosion behavior of materials. These methods involve applying small amplitude voltage or current perturbations to the material’s surface and measuring the resulting impedance or current response. By analyzing the changes in electrode potential and the corresponding electrochemical parameters, the corrosion resistance, passivation characteristics, and localized corrosion susceptibility of materials can be evaluated.
Overall, electrode potential measurements are an essential tool in corrosion testing and materials characterization. They provide valuable information about the corrosion behavior, corrosion rate, susceptibility to corrosion, and effectiveness of corrosion protection methods. By understanding the electrode potential of different materials in corrosive environments, researchers and engineers can make informed decisions about material selection, design, and corrosion mitigation strategies.
Analytical Techniques
Electrode potential plays a role in various analytical techniques, such as voltammetry and potentiometry. These techniques utilize the changes in electrode potential to analyze and quantify substances in solution, including metal ions, pollutants, and biological molecules. Understanding electrode potential is essential for controlling and predicting the behavior of electrodes in electrochemical systems. It has broad applications. Electrode potential plays a crucial role in various analytical techniques, where it is used to measure, detect, and analyze chemical species in samples. Electrochemical analytical techniques rely on the measurement of electrode potential to obtain information about the concentration, identity, and behavior of analytes. Here are some key analytical techniques that utilize electrode potential:
- Potentiometry: Potentiometry is a widely used electrochemical technique based on the measurement of electrode potential. It involves measuring the potential difference between a reference electrode and an indicator electrode in a solution. By exploiting the Nernst equation, the electrode potential can be related to the concentration of the analyte. Potentiometry is employed for pH measurement, ion concentration determination (e.g., in ion-selective electrodes), and titrations.
- Voltammetry: Voltammetry is an electroanalytical technique that involves applying a potential sweep or step to an electrode and measuring the resulting current. By varying the electrode potential, information about the redox behavior, electrochemical reactions, and analyte concentration can be obtained. Various voltammetric methods, such as cyclic voltammetry, differential pulse voltammetry, and square wave voltammetry, are used for qualitative and quantitative analysis of analytes, including metals, organic compounds, and biomolecules.
- Amperometry: Amperometry is an electroanalytical technique that measures the current flowing between an electrode and a counter electrode when a fixed potential is applied. The current is directly proportional to the concentration of the analyte being oxidized or reduced at the working electrode. Amperometry is commonly used for real-time monitoring of electroactive species, such as glucose, neurotransmitters, and pharmaceuticals, in fields like clinical diagnostics and environmental monitoring.
- Impedance Spectroscopy: Impedance spectroscopy is an electrochemical technique that measures the impedance response of an electrode-electrolyte interface over a range of frequencies. The impedance includes resistance (real part) and capacitance or inductance (imaginary part). By analyzing the impedance spectrum, valuable information about the interfacial properties, charge transfer kinetics, and analyte interactions can be obtained. Impedance spectroscopy finds applications in material characterization, corrosion analysis, and biosensing.
- Conductometry: Conductometry is an analytical technique that measures the electrical conductivity of a solution. By using conductivity cells with metal electrodes, the electrode potential can influence the conductivity measurement. The concentration of electrolytes or ionic species in a solution can be determined based on their conductivities. Conductometry is commonly used in chemical analysis, water quality assessment, and titrations.
- Ion-Selective Electrodes (ISE): Ion-selective electrodes are specialized electrodes that selectively respond to specific ions in a sample. They operate based on the principle of ion exchange between the sample solution and a membrane on the electrode surface. The potential difference generated is related to the concentration of the target ion. Ion-selective electrodes are used for the direct measurement of various ions, including pH, potassium, sodium, calcium, chloride, and many others.
In each of these analytical techniques, the electrode potential is a key parameter that influences the measurement and detection of analytes. By controlling and monitoring the electrode potential, researchers and analysts can obtain accurate and precise results. Calibration of the electrodes and careful control of experimental conditions are essential for obtaining reliable and reproducible measurements in analytical techniques utilizing electrode potential.