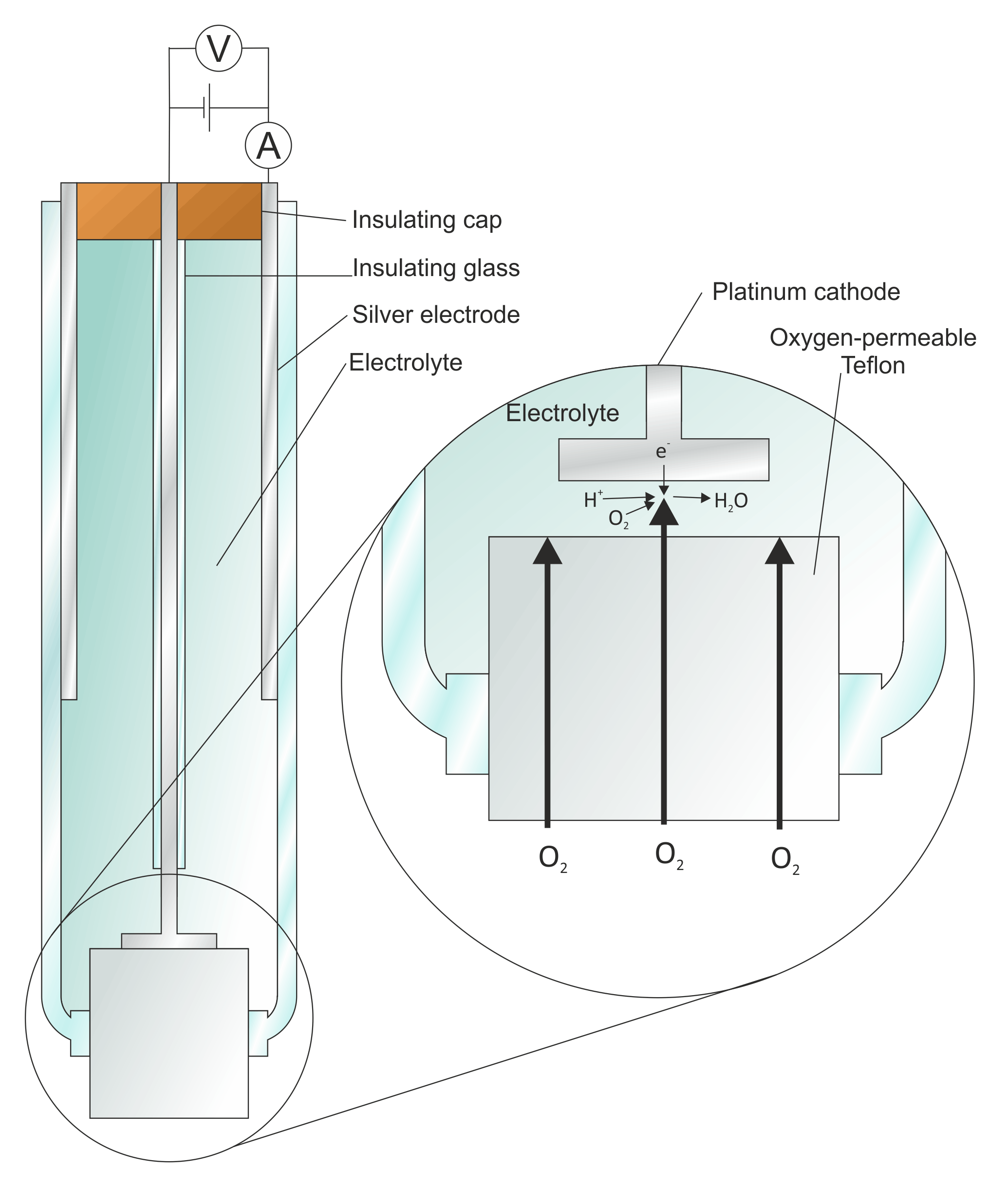
Introduction
In electrochemistry, there are several types of electrodes used to facilitate the flow of electrons and participate in various electrochemical reactions. These electrodes can be classified based on their composition and application. Here are some common types of electrodes:
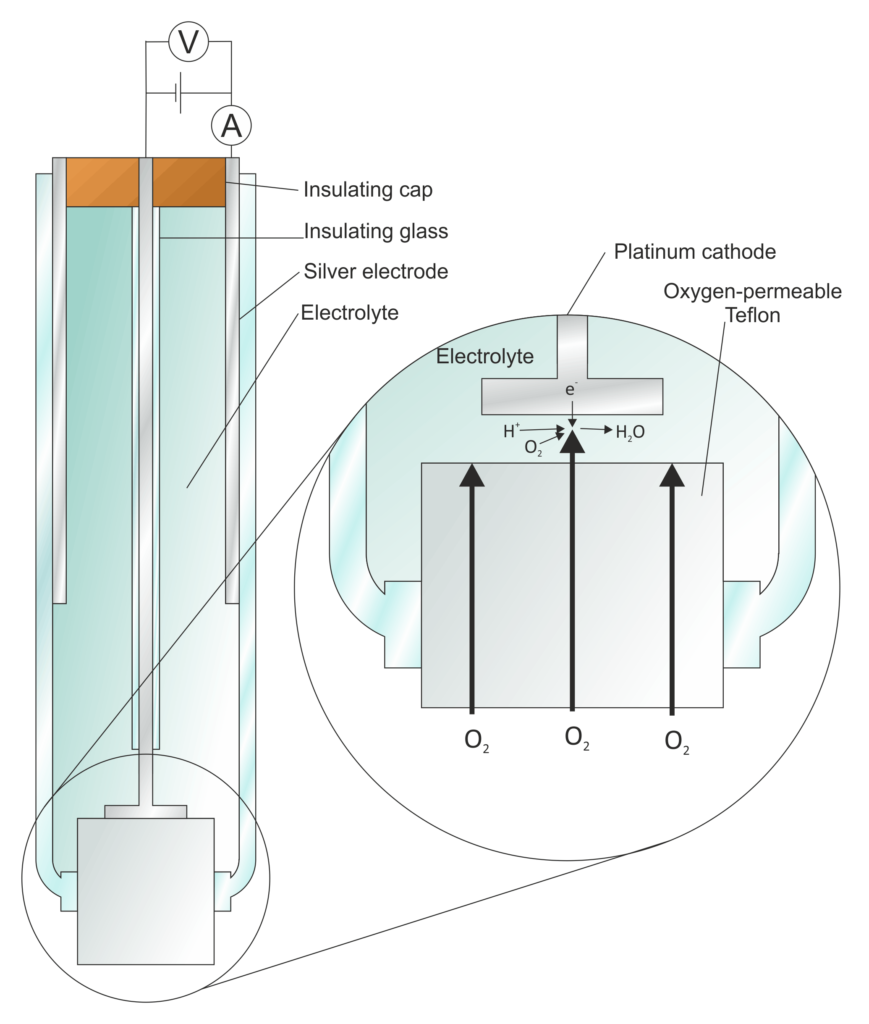
Types of electrodes
- Metal Electrodes: These are the most common type of electrodes and are typically made of a conductive metal or metal alloy. Examples include platinum, gold, silver, and stainless steel electrodes. Metal electrodes are widely used for various electrochemical processes and experiments.
- Reference Electrodes: Reference electrodes are used to establish a known electrochemical potential against which other electrodes can be measured. The potential of a reference electrode remains stable and does not change during the course of an experiment. Popular examples include the Standard Hydrogen Electrode (SHE), Silver/Silver chloride electrode (Ag/AgCl), and Calomel electrode (Hg/Hg2Cl2). Reference electrodes are a crucial component in electrochemical measurements and experiments. They provide a stable and known electrochemical potential against which the potential of other electrodes can be measured. The potential of a reference electrode remains constant and does not change during the course of an experiment. Here are some common types of reference electrodes used in electrochemistry:
- Standard Hydrogen Electrode (SHE): The Standard Hydrogen Electrode is one of the oldest and most widely used reference electrodes. It consists of a platinum electrode immersed in a solution of 1 M HCl with a stream of hydrogen gas bubbling through it. The electrode potential is defined as 0 volts at all temperatures.
- Silver/Silver Chloride Electrode (Ag/AgCl): The Ag/AgCl reference electrode is another popular choice in many electrochemical studies. It consists of a silver wire coated with silver chloride (AgCl) immersed in a potassium chloride (KCl) solution. The electrode potential is stable and close to +0.197 V versus the Standard Hydrogen Electrode.
- Calomel Electrode (Hg/Hg2Cl2): The calomel electrode is a mercury-based reference electrode. It comprises a mercury pool in contact with a paste of mercurous chloride (Hg2Cl2) and saturated potassium chloride (KCl) solution. The calomel electrode has a stable potential, typically around +0.241 V versus the Standard Hydrogen Electrode.
- Saturated Calomel Electrode (SCE): The SCE is a variation of the calomel electrode with a defined potential of +0.242 V versus the Standard Hydrogen Electrode at 25°C. It is widely used in electrochemical measurements and serves as a common reference in various applications.
- Silver/Silver Sulfate Electrode (Ag/Ag2SO4): This reference electrode consists of a silver wire coated with silver sulfate (Ag2SO4) immersed in a potassium sulfate (K2SO4) solution. It provides a stable potential, commonly around +0.070 V versus the Standard Hydrogen Electrode.
- Reference Electrode with a Salt Bridge: Some reference electrodes are connected to the test solution via a salt bridge to prevent direct mixing of the reference and sample solutions. The salt bridge contains an electrolyte (often KCl or KNO3) that allows for the flow of ions, maintaining electrical neutrality. Reference electrodes are essential for accurate and reproducible electrochemical measurements, enabling researchers to understand and study the behavior of various electrochemical systems. The choice of a specific reference electrode depends on the nature of the experiment, the electrolyte used, and the potential range of interest.
- Saturated Calomel Electrode (SCE): This is a specific type of reference electrode consisting of mercury and mercurous chloride in a saturated potassium chloride solution.
- Working Electrodes: These electrodes are the main focus of an electrochemical experiment. They participate in the redox reaction and are responsible for measuring the current or potential difference. Working electrodes can be made of various materials, such as platinum, gold, glassy carbon, and carbon nanotubes.
- Counter Electrodes: Counter electrodes complete the electrical circuit in electrochemical cells. They have a large surface area and are made from conductive materials like platinum or graphite. Counter electrodes are not directly involved in the reaction of interest but ensure efficient electron transfer between the working electrode and the electrolyte.
- Indicator Electrodes: Indicator electrodes are used to detect specific ions or substances in solution. They are coated with selective membranes or ion-specific sensors that respond to the presence of particular analytes.
- Gas-Sensing Electrodes: These electrodes are designed to measure the concentration of specific gases dissolved in the electrolyte solution. They are commonly used in gas sensors and gas detection devices.
- pH Electrodes: These electrodes are used to measure the pH of a solution. They consist of a glass membrane that responds to changes in hydrogen ion concentration.
- Ion-Selective Electrodes (ISE): Ion-selective electrodes are used to measure the concentration of specific ions in solution. They are equipped with ion-specific sensing elements that respond selectively to the target ion.
These are some of the most common types of electrodes used in electrochemistry. Each type serves a specific purpose in various electrochemical applications and plays a crucial role in the accurate measurement and study of electrochemical processes.
Here are a few more types of electrodes used in electrochemistry:
- Screen-Printed Electrodes (SPE): These are low-cost, disposable electrodes made by screen-printing conductive ink onto a non-conductive substrate, such as ceramic or polymer. SPEs are widely used in various applications, including environmental monitoring, point-of-care medical devices, and electrochemical sensors.
- Rotating Disk Electrode (RDE): An RDE is a specialized working electrode that rotates at a controlled speed. This type of electrode is commonly used in hydrodynamic studies and mass transport investigations, particularly in the study of electrode kinetics and electrocatalysis.
- Microelectrodes: Microelectrodes have small dimensions, typically on the micrometer scale, and are used to study electrochemical processes at very localized spots. They offer high spatial resolution and find applications in neuroscience, corrosion studies, and other fields requiring precise measurements.
- Bipolar Electrodes: These electrodes are designed to carry out electrolysis reactions without the need for an external power supply. A bipolar electrode consists of two or more conducting materials connected together in a single structure.
- Dropping Mercury Electrode (DME): A DME is a type of working electrode used in polarography and voltammetry. It consists of a small droplet of mercury that forms at the tip of a capillary. The mercury drop serves as both the working electrode and the conductor.
- Amalgam Electrodes: Amalgam electrodes are formed by combining mercury with another metal, typically a reactive metal like bismuth or antimony. These electrodes are useful in studies where mercury amalgamation is needed.
- Wireless Electrodes: Wireless electrodes are a modern development in electrochemistry. They utilize wireless communication technology to transmit data from the electrode to a connected device, reducing the need for physical connections and allowing for more flexible experimental setups.
- Photoelectrodes: Photoelectrodes are light-sensitive electrodes that can generate an electrical current when exposed to light. They are crucial components in photoelectrochemical cells used for solar energy conversion and environmental remediation.
- Carbon Paste Electrodes (CPE): Carbon paste electrodes consist of a mixture of graphite powder and a binding agent (such as mineral oil or paraffin wax). They are versatile and can be modified with various materials to serve different analytical purposes.
- Flow-through Electrodes: These electrodes are designed for continuous flow systems, such as flow-through electrochemical cells. They facilitate rapid exchange of the analyte and are commonly used in online monitoring and process control applications.
- Pencil Electrodes: Pencil electrodes are simple and economical electrodes made by inserting a pencil lead (graphite) into a holder. They are often used in educational settings for introductory electrochemistry experiments.
- Disk Electrodes: Disk electrodes are flat, circular electrodes with a defined surface area. They are commonly used in techniques like cyclic voltammetry and chronoamperometry to study redox reactions and measure reaction kinetics.
- Wire Electrodes: Wire electrodes are simple electrodes made from conductive wire, such as platinum or gold wire. They can be used in various configurations and are particularly useful for small-scale experiments and applications requiring flexibility.
- Interdigitated Electrodes (IDEs): Interdigitated electrodes consist of sets of alternating fingers or comb-like structures, where the fingers of one set are interleaved with the other set. IDEs are used in various applications, including impedance spectroscopy and biosensing, due to their unique electrical properties.
- Screen-Printed Carbon Electrodes (SPCE): Similar to screen-printed electrodes, SPCEs are made by screen-printing carbon-based ink onto a substrate. They find applications in various fields, such as environmental monitoring, food analysis, and pharmaceutical research.
- Three-Electrode System: The three-electrode system is a common experimental setup in electrochemistry, comprising a working electrode, a reference electrode, and a counter electrode. This configuration allows for precise control and accurate measurements in many electrochemical experiments.
- Gas Diffusion Electrodes: Gas diffusion electrodes are used in electrochemical devices that involve gas-liquid reactions, such as fuel cells and certain electrolyzers. They facilitate the efficient mass transport of gas molecules to the electrode surface.
- Flow Electrodes: Flow electrodes are designed to work in conjunction with a flowing electrolyte solution. They are commonly used in flow-through electrochemical cells, where the continuous flow enhances mass transport and reduces fouling.
- Optical Electrodes: Optical electrodes utilize optical fibers or other light-sensitive elements to monitor changes in the optical properties of the electrode material during electrochemical reactions. They find applications in biosensing and corrosion studies.
- Ring-Disk Electrode (RDE): A ring-disk electrode consists of a central disk electrode surrounded by a ring-shaped electrode. This setup allows simultaneous measurements of the faradaic current at the disk and the diffusion-controlled current at the ring, making it useful for studying electrode reactions with coupled chemical reactions.
- Nanoelectrodes: Nanoelectrodes have dimensions in the nanometer range, typically 1-100 nm. They offer extremely high spatial resolution and are used for studying electrochemical processes at the nanoscale, such as single-molecule reactions and nanoscale surface properties.
- Sacrificial Electrodes: Sacrificial electrodes are consumed during an electrochemical reaction, allowing other species to be reduced or oxidized. They are commonly used in corrosion protection systems, electroplating, and metal-ion removal processes.
- Dual-Electrode System: A dual-electrode system involves two working electrodes in an electrochemical cell. It allows for simultaneous monitoring of two different electrochemical processes or the comparison of different electrode materials.
These are some additional types of electrodes used in electrochemistry, and each type serves specific purposes in various research and industrial applications. The selection of the appropriate electrode depends on the nature of the electrochemical study and the desired experimental conditions. Each type of electrode has its unique characteristics and advantages, making them suitable for different experimental setups and applications in electrochemistry. Researchers and scientists select the appropriate electrode based on the specific requirements of their studies and measurements.
Top 10 books on the electrode
here are ten highly regarded books that cover various aspects of electrodes and electrochemistry:
- “Electrochemical Methods: Fundamentals and Applications” by Allen J. Bard and Larry R. Faulkner – This comprehensive textbook is a classic in the field of electrochemistry, covering fundamental principles and techniques related to electrode processes and applications.
- “Electrode Dynamics” by Andrei N. Frumkin – This book delves into the theory of electrode processes, including the kinetics of electrochemical reactions and the behavior of different types of electrodes.
- “Modern Electrochemistry 2A: Fundamentals of Electrodics” by John O’M. Bockris, Amulya K.N. Reddy, and Maria E. Gamboa-Aldeco – This volume is part of the renowned “Modern Electrochemistry” series and provides a thorough introduction to electrodics and electrode reactions.
- “Electroanalytical Chemistry: A Series of Advances” edited by Allen J. Bard – This book is part of a series that covers recent advances and cutting-edge research in the field of electroanalytical chemistry, including electrode development and applications.
- “Electrochemistry of Nucleic Acids and Proteins: Towards Electrochemical Sensors for Genomics and Proteomics” by Goretti Requicha Ferreira and Maria Gabriela Almeida – This book explores the electrochemical properties and applications of nucleic acids and proteins, focusing on the development of electrochemical sensors for genomics and proteomics.
- “Electrochemical Sensors, Biosensors and their Biomedical Applications” edited by Xueji Zhang – This book covers the principles and applications of electrochemical sensors and biosensors, including their use in biomedical and clinical settings.
- “Electrodes: Advances in Materials and Interface Design” edited by Piotr Kula and Adam C. Granger – This book focuses on advances in electrode materials and design, with contributions from experts in the field.
- “Nanoscale Electrochemistry” edited by Oleg V. Tolstogouzov – This book explores the electrochemical properties of nanostructured materials and their applications in various fields, including energy storage and conversion.
- “Carbon Electrochemical Supercapacitors: Fundamentals and Applications” by Franck Dolhem and Patrice Simon – This book provides an in-depth understanding of carbon-based supercapacitor technology, including the electrochemistry of carbon electrodes.
- “Electrochemical Impedance Spectroscopy and its Applications” by Andrzej Lasia – This book focuses on the theory and applications of electrochemical impedance spectroscopy, a powerful technique used to study the electrical properties of electrodes and interfaces.
Please note that the field of electrochemistry is continually evolving, and new books and research may have emerged since my last update. Be sure to check for the latest publications and reviews to find the most relevant and up-to-date resources on electrodes and electrochemistry.