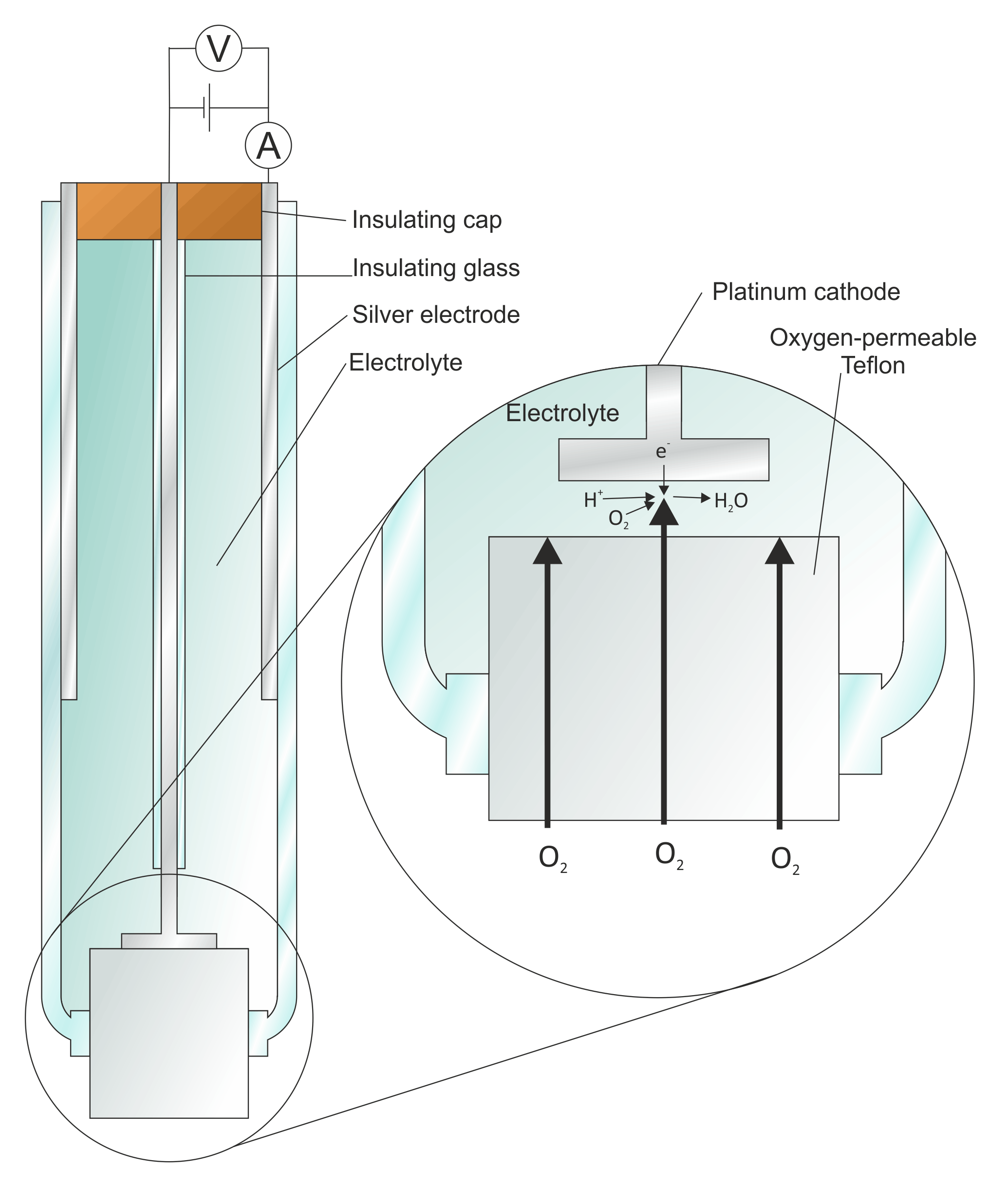
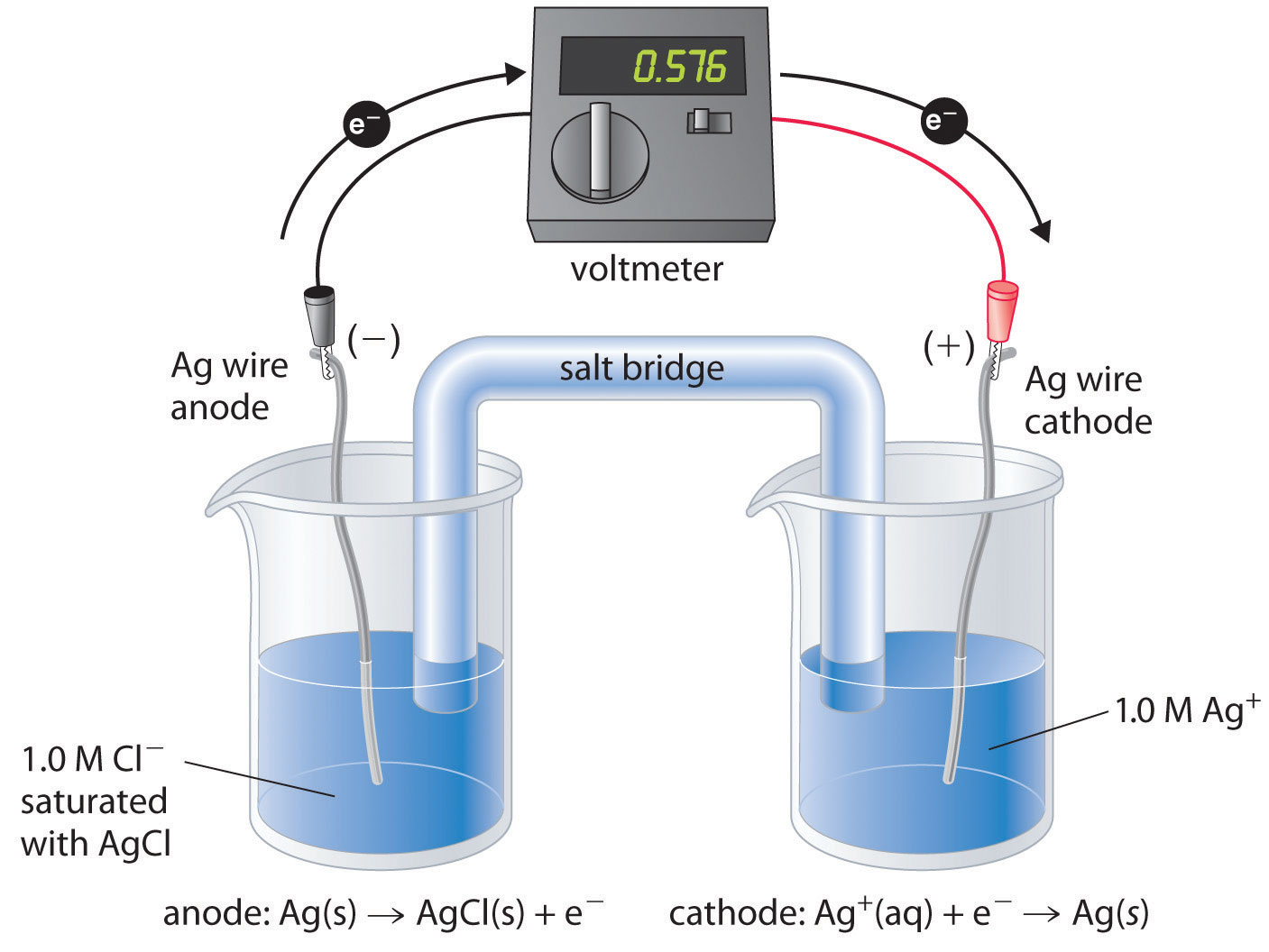
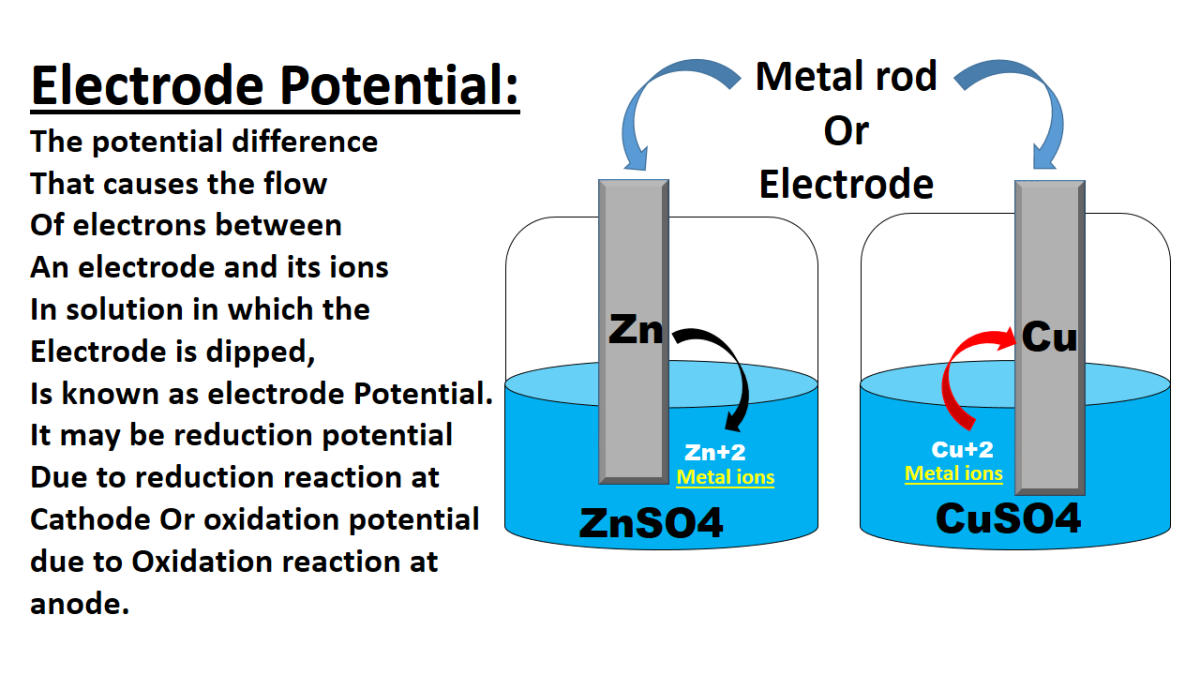
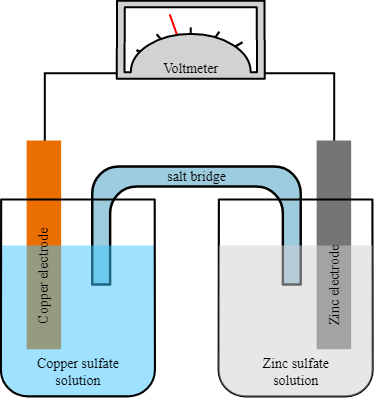
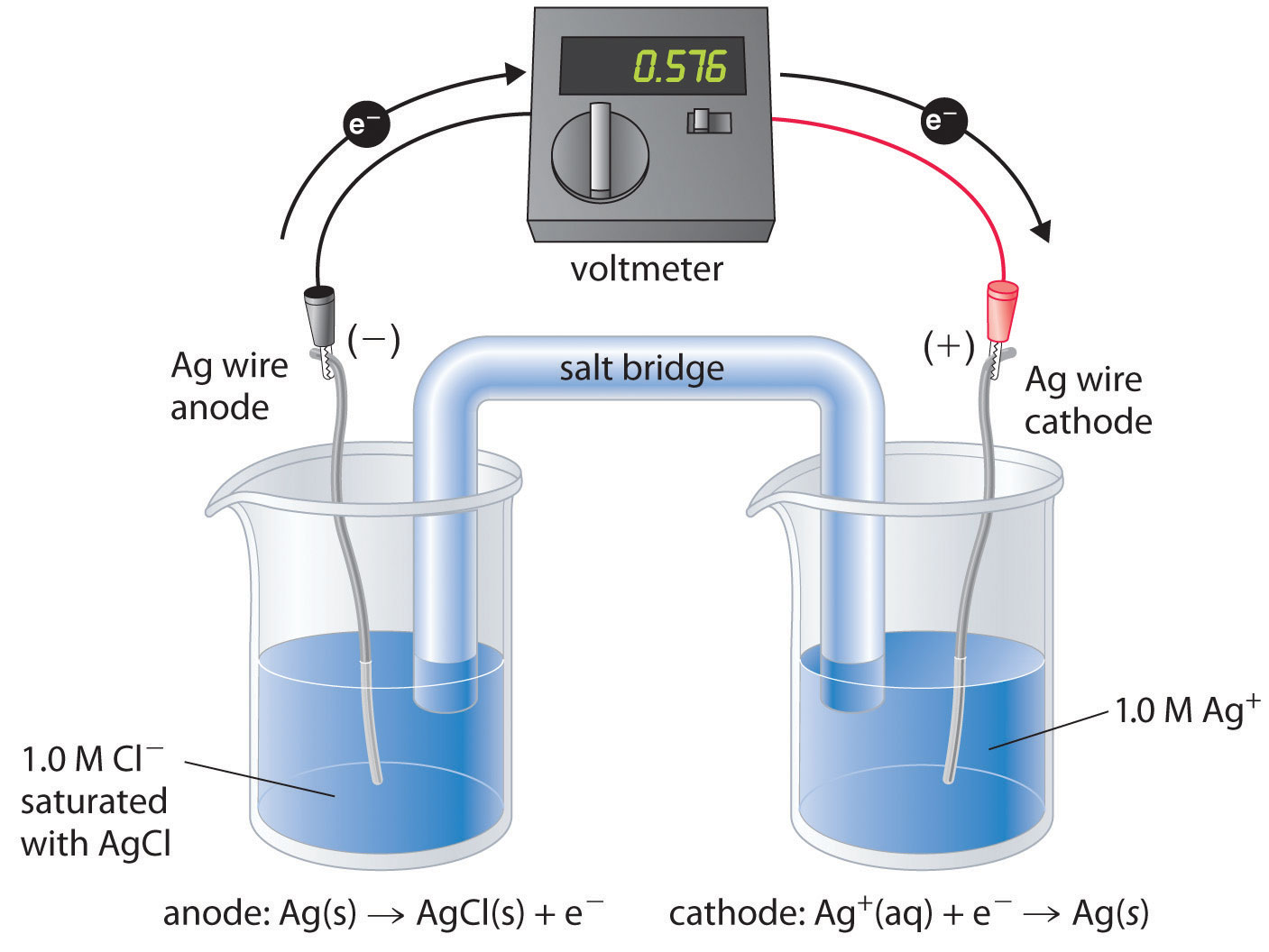
Types of Electrodes in Electrochemistry
Introduction In electrochemistry, there are several types of electrodes used to facilitate the flow of electrons and participate in various electrochemical reactions. These electrodes can be classified based on their …