Redox Flow Batteries
Introduction
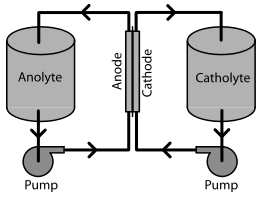
Redox flow batteries (RFBs) are a type of rechargeable electrochemical energy storage device that use two tanks of liquid electrolyte to store energy. Unlike traditional batteries that store energy in solid electrodes, RFBs use liquid electrolytes to store energy in a reversible redox reaction.
The basic design of an RFB consists of two tanks filled with liquid electrolytes, which are separated by a membrane. The electrolytes contain chemical species that can be oxidized or reduced during the charging or discharging process. When charging, one tank of electrolyte is oxidized, and the other is reduced, causing electrons to flow through an external circuit. During discharge, the process is reversed, and the electrons flow back through the external circuit to produce electricity.
One of the primary advantages of RFBs is their scalability. Because the energy is stored in liquid electrolytes, RFBs can be designed with larger storage capacities than traditional batteries without the same risk of overheating or thermal runaway. This makes RFBs particularly well-suited for grid-scale energy storage applications, where large amounts of energy need to be stored and released on demand.
There are several different types of RFBs, each with its own advantages and limitations. The most common types of RFBs are the vanadium redox flow battery (VRFB), the zinc-bromine flow battery, and the iron-chromium flow battery.
Vanadium Redox Flow Batteries (VRFB):
VRFBs are the most common type of RFB and use vanadium ions in different oxidation states (V2+ and V3+ in one electrolyte and V4+ and V5+ in the other) to store energy. The two electrolytes are separated by a proton exchange membrane, which allows protons to pass through but prevents the two electrolytes from mixing.
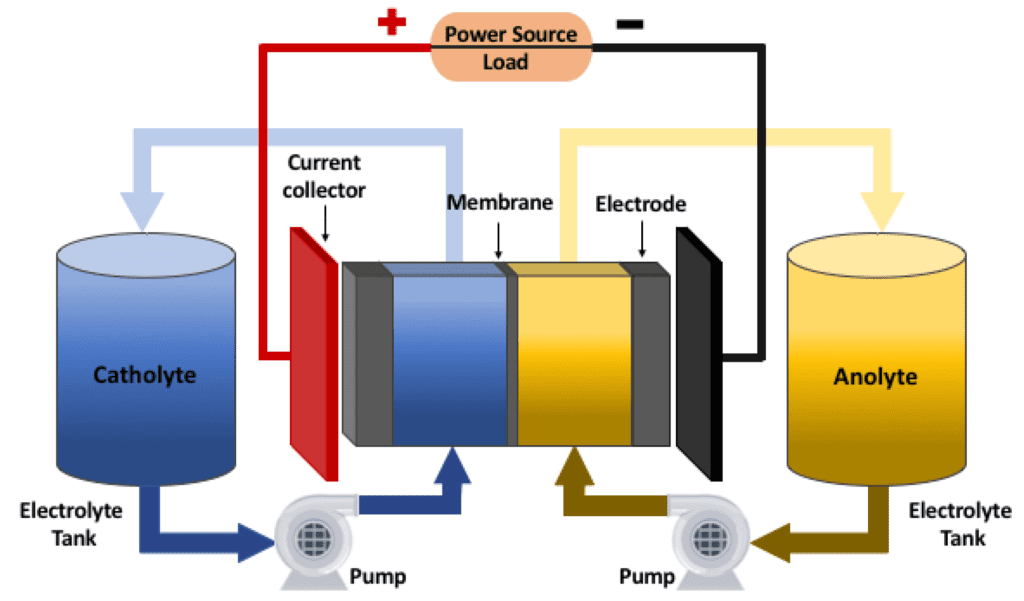
Vanadium Redox Flow Batteries (VRFBs) are a type of rechargeable battery that use vanadium ions in different oxidation states to store and release electrical energy. VRFBs consist of two tanks of vanadium electrolyte, separated by a membrane, and two sets of electrodes. The electrolytes in each tank are in different oxidation states, typically V(II) and V(III) in one tank and V(IV) and V(V) in the other. When the battery is charging, electrons are transferred from one electrode to the other, causing a change in the oxidation state of the vanadium ions. During discharge, the electrons flow back through an external circuit, generating electricity.
VRFBs have several advantages over other types of batteries. They have a longer lifespan, can be discharged and recharged many times without loss of capacity, and have a relatively high energy density. They can also be easily scaled up or down depending on the energy storage needs. Additionally, because the electrolytes are stored in separate tanks, VRFBs have a low risk of fire or explosion.
VRFBs are currently used in several applications, including renewable energy storage and backup power for telecommunications and data centers. However, they are still relatively expensive compared to other types of batteries, and research is ongoing to improve their performance and reduce costs.
One of the advantages of VRFBs is their ability to cycle for a large number of charge and discharge cycles without losing capacity. VRFBs also have relatively high energy efficiency, with round-trip efficiencies ranging from 75% to 85%. However, VRFBs have a relatively low energy density, meaning they require a large amount of space to store the same amount of energy as a traditional battery.
Zinc-Bromine Flow Batteries:
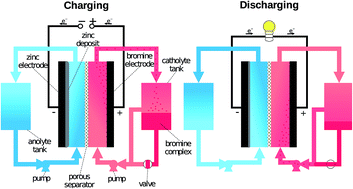
Zinc-bromine flow batteries use zinc and bromine ions in different oxidation states to store energy. The zinc and bromine electrolytes are separated by a membrane that allows ions to pass through but prevents the two electrolytes from mixing.
RSC Adv., 2016,6, 110548-110556
Zinc-bromine flow batteries have a higher energy density than VRFBs, meaning they can store more energy in the same amount of space. They also have a longer cycle life than VRFBs, with round-trip efficiencies ranging from 75% to 85%. However, zinc-bromine flow batteries require a more complex system to control the chemical reactions between the electrolytes and can be prone to performance degradation over time.
Iron-Chromium Flow Batteries
Iron-chromium flow batteries use iron and chromium ions in different oxidation states to store energy. The two electrolytes are separated by a membrane that allows ions to pass through but prevents the two electrolytes from mixing.
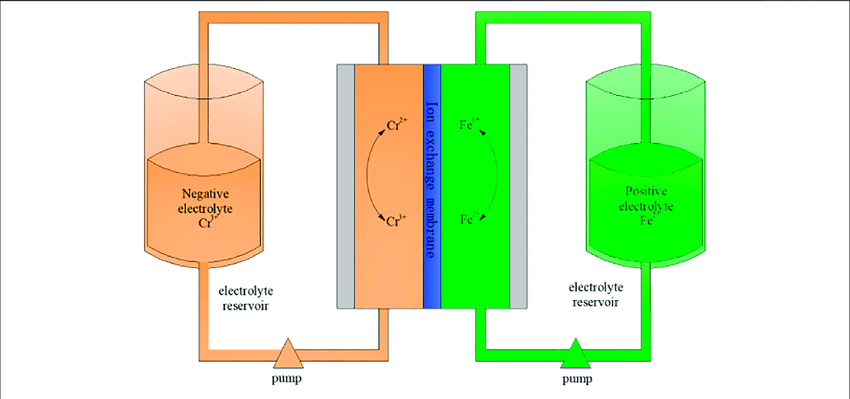
Photo Credit: X.-M. Wang et al
One of the advantages of iron-chromium flow batteries is their relatively low cost compared to other types of RFBs. They also have a high energy density and a relatively long cycle life, with round-trip efficiencies ranging from 70% to 80%. However, iron-chromium flow batteries have a lower power density than other types of RFBs, meaning they may not be well-suited for applications that require high power output.
Applications of Redox Flow Batteries:
Redox flow batteries (RFBs) have a wide range of potential applications due to their scalability, long cycle life, and ability to discharge energy for extended periods of time. Some of the most promising applications of RFBs include:
- Grid-scale energy storage: RFBs are well-suited for grid-scale energy storage applications, where large amounts of energy need to be stored and released on demand. RFBs can help utilities to balance the supply and demand of electricity, particularly as renewable energy sources such as wind and solar become more prevalent.
- Backup power: RFBs can be used to provide backup power for critical infrastructure, such as hospitals and data centers. RFBs can be designed to provide energy for extended periods of time, making them a reliable source of backup power during power outages.
- Electric vehicles: RFBs have the potential to be used in electric vehicles, particularly for large vehicles such as buses and trucks. RFBs could provide a longer driving range and faster charging times than traditional lithium-ion batteries, while also reducing the risk of thermal runaway.
- Remote power: RFBs can be used to provide power in remote areas, such as off-grid communities or military bases. RFBs can store energy from renewable sources such as solar and wind, providing a reliable source of power even in remote locations.
- Microgrids: RFBs can be used to support microgrids, which are small-scale power systems that can operate independently or in conjunction with the main power grid. RFBs can help to balance the supply and demand of electricity in microgrids, particularly as renewable energy sources become more prevalent.
- Energy arbitrage: RFBs can be used for energy arbitrage, which involves buying electricity when prices are low and selling it when prices are high. RFBs can store energy when prices are low and discharge it when prices are high, providing a way to generate revenue from energy storage.
Overall, the applications of RFBs are diverse and growing. As the technology continues to improve and costs decrease, RFBs are expected to become an increasingly important part of the energy storage landscape.
Fundamental of Redox Flow Batteries:
Redox flow batteries (RFBs) are a type of rechargeable battery that use two liquid electrolytes containing redox-active species, separated by an ion-permeable membrane, to store and release energy. During charging, the electrolyte in one tank is oxidized, while the electrolyte in the other tank is reduced, causing the redox-active species to undergo a chemical reaction that produces electrons. The electrons are then sent through an external circuit to generate electrical energy. During discharge, the process is reversed, and the redox-active species are restored to their original oxidation states, producing electrical energy that can be used to power electrical devices or stored for later use.
The basic components of an RFB include two tanks containing liquid electrolytes, an ion-permeable membrane that separates the two electrolytes, and two electrodes connected to an external circuit. The redox-active species in the electrolytes can be metal ions, such as vanadium or iron, or organic compounds, such as quinones or viologens. The choice of redox-active species depends on the specific requirements of the application, such as the desired energy density, cycle life, and cost.
The energy density of an RFB is determined by the concentration of the redox-active species in the electrolyte and the volume of the electrolyte. Higher concentrations of redox-active species lead to higher energy densities, but can also increase the cost and complexity of the system. The cycle life of an RFB is determined by the stability of the redox-active species and the membrane, as well as the efficiency of the electrochemical reactions. In general, RFBs have longer cycle lives than traditional batteries, making them well-suited for applications that require frequent charging and discharging.
One of the key advantages of RFBs is their scalability. Because the energy is stored in liquid electrolytes, RFBs can be designed with larger storage capacities than traditional batteries without the same risk of overheating or thermal runaway. This makes RFBs particularly well-suited for grid-scale energy storage applications, where large amounts of energy need to be stored and released on demand.
There are several different types of RFBs, each with its own advantages and limitations. The most common types of RFBs are the vanadium redox flow battery (VRFB), the zinc-bromine flow battery, and the iron-chromium flow battery. VRFBs use vanadium ions in different oxidation states to store energy, while zinc-bromine flow batteries and iron-chromium flow batteries use zinc and bromine ions and iron and chromium ions, respectively. Each type of RFB has unique characteristics that make it well-suited for certain applications.
RFBs have several advantages over traditional batteries, including their scalability, long cycle life, and ability to discharge energy for extended periods of time. However, RFBs also have some limitations, such as their lower energy density compared to traditional batteries and the cost and complexity of the system. Despite these limitations, RFBs are becoming increasingly important for a wide range of applications, from grid-scale energy storage to backup power and electric vehicles.
Benefits of redox Flow Batteries:
Redox flow batteries (RFBs) offer several benefits compared to traditional battery technologies, including:
- Scalability: RFBs are highly scalable, and can be designed with larger storage capacities than traditional batteries without the same risk of overheating or thermal runaway. This makes RFBs particularly well-suited for grid-scale energy storage applications, where large amounts of energy need to be stored and released on demand.
- Long cycle life: RFBs have longer cycle lives than traditional batteries, making them well-suited for applications that require frequent charging and discharging. This can help to reduce the lifetime cost of the system and make it more economically feasible over the long term.
- High efficiency: RFBs have high round-trip efficiencies, meaning that they can store and release energy with minimal losses. This helps to maximize the amount of energy that can be stored and used, while minimizing waste.
- Safe operation: RFBs are generally considered to be safe and reliable, with a lower risk of thermal runaway or other safety issues compared to some traditional battery technologies. This makes RFBs well-suited for applications where safety is a primary concern, such as in electric vehicles or critical infrastructure.
- Flexibility: RFBs can use a variety of different redox-active species, including metal ions and organic compounds, allowing them to be tailored to specific applications and requirements. This makes RFBs more versatile than traditional battery technologies, which are often limited to a specific chemistry.
- Sustainability: RFBs can use renewable energy sources such as solar and wind to charge, making them a more sustainable energy storage solution than traditional batteries that rely on fossil fuels. Additionally, RFBs can be recycled or reused, reducing the environmental impact of the system over its lifetime.
Overall, the benefits of RFBs make them an attractive option for a wide range of applications, from grid-scale energy storage to electric vehicles and backup power. As the technology continues to improve and costs decrease, RFBs are expected to become an increasingly important part of the energy storage landscape.
Limitation of Redox Flow Batteries:
While redox flow batteries (RFBs) offer several advantages over traditional battery technologies, there are also some limitations and challenges associated with their use. These include:
- Lower energy density: RFBs typically have lower energy densities than traditional batteries, meaning that they require more space to store the same amount of energy. This can be a disadvantage in some applications where space is limited, such as in portable electronics or electric vehicles.
- Lower power density: RFBs also typically have lower power densities than traditional batteries, meaning that they may not be able to deliver energy as quickly. This can be a disadvantage in applications that require high power outputs, such as in electric vehicles or grid-scale energy storage.
- Cost: RFBs can be more expensive than traditional battery technologies, particularly at smaller scales. While costs have been decreasing over time, RFBs may not be cost-effective for all applications, particularly those with low energy requirements.
- Limited cycle life: While RFBs have longer cycle lives than traditional batteries, they can still degrade over time with use. This can limit the lifetime of the system and require more frequent maintenance or replacement.
- Corrosion: The materials used in RFBs, particularly those in contact with the electrolyte, can be prone to corrosion. This can limit the lifetime of the system and require more frequent maintenance or replacement.
- Environmental impact: While RFBs can be more sustainable than traditional battery technologies, they still have environmental impacts associated with their manufacture and disposal. Additionally, some of the materials used in RFBs can be toxic or hazardous if not handled properly.
Overall, while RFBs offer several advantages over traditional battery technologies, their limitations and challenges must be carefully considered when evaluating their use in specific applications. As the technology continues to improve and costs decrease, RFBs are expected to become more competitive with traditional battery technologies and find new applications where their unique advantages can be leveraged.
Future of Redox Flow Batteries:
The future of redox flow batteries (RFBs) looks promising, as these technologies offer several advantages over traditional battery technologies and are well-suited for a wide range of energy storage applications. Here are some key areas where RFBs are expected to make an impact in the coming years:
- Grid-scale energy storage: One of the primary applications for RFBs is in grid-scale energy storage, where they can help to balance supply and demand and enable the integration of intermittent renewable energy sources like solar and wind. As the cost of RFBs continues to decrease, they are expected to become increasingly competitive with traditional battery technologies for grid-scale applications.
- Backup power: RFBs are also well-suited for backup power applications, as they can store large amounts of energy and release it on demand with high efficiency. This makes them ideal for applications where reliable backup power is critical, such as hospitals, data centers, and other critical infrastructure.
- Electric vehicles: While RFBs are not yet widely used in electric vehicles, they offer several advantages over traditional lithium-ion batteries, including longer cycle lives and safer operation. As the cost of RFBs decreases and their energy and power densities improve, they may become a more attractive option for electric vehicle manufacturers.
- Remote and off-grid applications: RFBs are ideal for remote and off-grid applications, where access to reliable and sustainable energy sources is limited. This includes applications such as rural electrification, off-grid telecommunications, and military installations.
- Energy arbitrage: RFBs can also be used for energy arbitrage, which involves buying low-cost energy when it is available and storing it for use during peak demand periods. This can help to reduce energy costs and improve the efficiency of the energy system.
Overall, the future of RFBs looks bright, as these technologies offer several advantages over traditional battery technologies and are well-suited for a wide range of energy storage applications. As research and development continue, and the cost and performance of RFBs continue to improve, they are expected to become an increasingly important part of the energy storage landscape.
Top 10 books on Redox Flow Batteries
Here are 10 books on Redox Flow Batteries that you may find helpful:
- “Redox Flow Batteries: Fundamentals and Applications” by Wei Wang, Yanwei Ma, and Lei Zhang
- “Redox Flow Batteries: Principles, Technologies and Applications” by Sagarika Mukhopadhyay and Keka Ojha
- “Redox Flow Batteries: Energy Storage for a Sustainable Future” by Ulf Bossel
- “Electrochemical Energy Storage for Renewable Sources and Grid Balancing” edited by Patrick T. Moseley, Jürgen Garche, and Chris Dyer
- “Redox Flow Batteries: A Review” by Partha P. Mukherjee and Brian S. Pivovar
- “Handbook of Energy Storage” edited by Patrick T. Moseley, Jürgen Garche, and Chris Dyer
- “Flow Batteries: Fundamentals, Applications, and Technologies” by Bahman Zohuri and Patrick T. Moseley
- “Redox Flow Batteries for Energy Storage” by Doris Segets, Tomás P. González, and Christian Kuss
- “Redox Flow Batteries: Advances, Challenges, and Opportunities” edited by Chuanxiang Zhang and Xiuling Zhang
- “Vanadium Redox Flow Batteries: An In-Depth Guide to Design, Performance, and Operation” by Andrew A. Hannah and Gareth W. Johnson
These books cover a wide range of topics related to Redox Flow Batteries, including their principles, design, performance, and applications.
