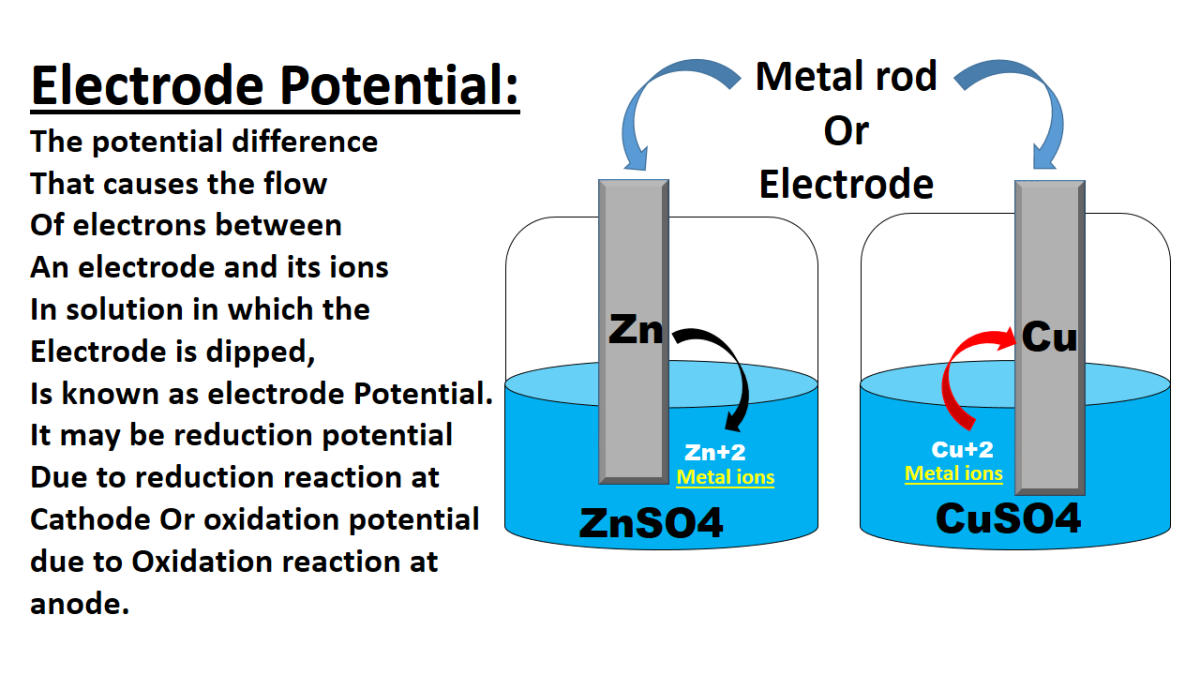
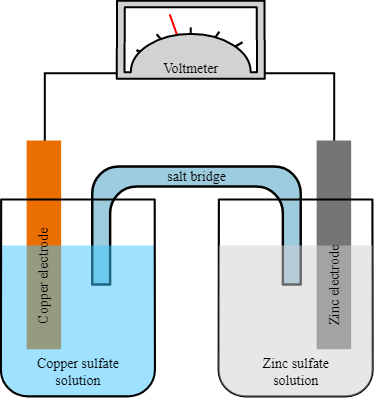
What is Electrode Potential and their Applications
Introduction Electrode potential, also known as electrode potential difference or electrode potential difference, is a measure of the tendency of an electrode to gain or lose electrons in an electrochemical …