Introduction
Electrolysis is an electrochemical process that uses an electric current to drive a non-spontaneous chemical reaction. It involves the decomposition of an electrolyte into its constituent elements or ions through the application of an external electric field. Electrolysis is widely used in various industrial processes, scientific research, and practical applications.
Electrolysis is a fundamental process in electrochemistry that involves the use of an electric current to induce a non-spontaneous chemical reaction. It is a powerful tool that allows for the transformation of compounds into their constituent elements or ions. Electrolysis has a wide range of applications in industry, scientific research, and everyday life. In this article, we will provide an in-depth introduction to electrolysis, including its principles, historical background, and key applications.
At its core, electrolysis relies on the principles of redox reactions and the movement of ions in the presence of an electric field. It takes place within an electrolytic cell, which consists of two electrodes—an anode and a cathode—immersed in an electrolyte solution. The anode is the positive electrode, while the cathode is the negative electrode.
During electrolysis, an external power source, such as a battery or power supply, is connected to the anode and cathode, creating an electric field. This electric field causes the migration of ions within the electrolyte solution toward their respective electrodes. The positively charged ions (cations) migrate toward the cathode, while the negatively charged ions (anions) migrate toward the anode.
At the anode, oxidation takes place. The anions from the electrolyte solution accept electrons released by the anode, leading to the formation of neutral atoms or molecules. At the cathode, reduction occurs. The cations from the electrolyte solution donate electrons to the cathode, resulting in the formation of neutral atoms or molecules.
The overall electrolysis reaction is determined by the specific electrolyte used and the desired outcome. It involves the sum of the anode and cathode reactions. The choice of electrolyte and the operating conditions, such as temperature and concentration, play a crucial role in determining the efficiency and feasibility of the electrolysis process.
Historical Background
The discovery and understanding of electrolysis can be traced back to the late 18th and early 19th centuries. The foundations were laid by several notable scientists, including Alessandro Volta, Michael Faraday, and Humphry Davy.
In 1800, Alessandro Volta invented the first true battery, known as the Voltaic Pile. This invention provided a reliable source of continuous electrical current, which enabled further exploration of electrical phenomena. Volta’s battery played a pivotal role in the development of electrochemical theories and the understanding of electrolysis.
Michael Faraday, in the 1830s, conducted extensive experiments on electrolysis and made significant contributions to the field. He formulated laws that govern the quantitative aspects of electrolysis, such as Faraday’s laws of electrolysis. These laws established a relationship between the amount of substance deposited or liberated during electrolysis and the amount of electric charge passed through the electrolyte.
Humphry Davy, a contemporary of Faraday, made notable discoveries in electrolysis. In 1807, Davy isolated several alkali and alkaline earth metals using electrolysis, including potassium, sodium, calcium, and magnesium. This marked a breakthrough in the field and showcased the power of electrolysis in the isolation and extraction of elements.
Principles of Electrolysis
Electrolysis is based on the principles of redox reactions and the movement of ions in an electric field. The process occurs in an electrolytic cell, which consists of two electrodes (anode and cathode) and an electrolyte solution.
- Anode: The anode in an electrolytic cell is the positive electrode. It attracts negatively charged ions (anions) from the electrolyte solution. At the anode, oxidation takes place, leading to the loss of electrons.
- Cathode: The cathode in an electrolytic cell is the negative electrode. It attracts positively charged ions (cations) from the electrolyte solution. At the cathode, reduction takes place, involving the gain of electrons.
- Electrolyte: The electrolyte in an electrolytic cell is a solution or molten compound that contains ions. It enables the conduction of electricity and facilitates the movement of ions between the electrodes.
- External Power Source: Electrolysis requires an external power source, such as a battery or power supply, to provide the necessary electric current. The power source supplies electrons to the cathode and withdraws electrons from the anode.
Process of Electrolysis
During electrolysis, the following steps occur:
- Electrolyte Dissociation: When an electric current is applied to the electrolyte solution, it dissociates into its constituent ions. The positive ions migrate toward the cathode, and the negative ions migrate toward the anode.
- Anode Reaction: At the anode, oxidation occurs, resulting in the release of electrons. The anions from the electrolyte accept these electrons, forming neutral atoms or molecules. For example, during the electrolysis of water, oxygen gas is produced at the anode.
2H₂O(l) -> O₂(g) + 4H⁺(aq) + 4e⁻
- Cathode Reaction: At the cathode, reduction takes place, involving the acceptance of electrons. The cations from the electrolyte donate these electrons, forming neutral atoms or molecules. For example, during the electrolysis of water, hydrogen gas is produced at the cathode.
4H⁺(aq) + 4e⁻ -> 2H₂(g)
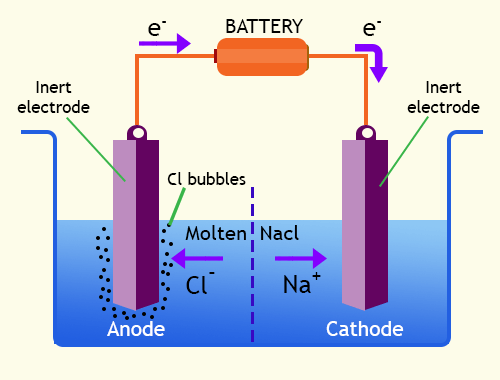
- Overall Electrolysis Reaction: The overall electrolysis reaction is determined by the specific electrolyte and the desired outcome. It involves the sum of the anode and cathode reactions. For example, the electrolysis of sodium chloride (NaCl) produces chlorine gas (Cl₂) at the anode and hydrogen gas (H₂) at the cathode.
2NaCl(aq) -> 2Na⁺(aq) + Cl₂(g) + 2e⁻ (anode reaction)
2H⁺(aq) + 2e⁻ -> H₂(g) (cathode reaction)
Applications of Electrolysis: Electrolysis has numerous practical applications, including:
- Electroplating: Electrolysis is widely used in electroplating processes to deposit a layer of metal onto a surface. This technique is employed to enhance the appearance, durability, and corrosion resistance of objects, such as jewelry, automobile parts, and household items.
Electroplating: Principles, Process, and Applications
Electroplating is a widely used electrochemical process that involves the deposition of a layer of metal onto a surface. It provides enhanced aesthetics, improved corrosion resistance, and increased durability to a wide range of objects. This article provides an in-depth overview of electroplating, including its principles, the electroplating process, and its applications across various industries.
The principles underlying electroplating are based on the principles of electrolysis and redox reactions. Electroplating utilizes an electrolytic cell that consists of an anode, a cathode, and an electrolyte solution.
- Anode: The anode in electroplating is the metal electrode that provides the source of metal ions to be deposited. It typically consists of the metal that will form the coating on the object being electroplated.
- Cathode: The cathode is the object to be electroplated. It is connected to the negative terminal of the power source and serves as the site where metal ions are reduced and deposited.
- Electrolyte Solution: The electrolyte solution contains metal ions of the coating material, which allows for the replenishment of metal ions at the cathode during the electroplating process. The solution may also contain various additives to improve the plating quality and performance.
- External Power Source: An external power source, such as a direct current power supply, is connected to the anode and cathode. It supplies the necessary electric current for the electroplating process to occur.
Electroplating Process: The electroplating process involves several steps to achieve a high-quality and uniform metal coating on the object being plated:
- Preparation: The object to be plated, known as the substrate, is thoroughly cleaned to remove any dirt, oils, or oxides from the surface. This step ensures good adhesion and quality of the plated metal layer.
- Electrolyte Preparation: The electrolyte solution is prepared by dissolving a salt of the desired metal in a suitable solvent. The concentration and composition of the electrolyte solution are carefully controlled to achieve the desired plating results.
- Plating Bath Setup: The electrolyte solution is placed in a plating bath, and the anode (consisting of the metal to be plated) and cathode (object to be plated) are immersed in the solution. They are positioned in such a way that ensures a uniform distribution of metal ions and a consistent plating process.
- Electroplating Process: When the power source is switched on, an electric current flows through the circuit. Metal cations from the anode dissolve into the electrolyte solution, while metal cations from the solution are reduced and deposited onto the cathode. This deposition occurs layer by layer, gradually forming the desired metal coating on the substrate.
- Post-Treatment: After the desired thickness and quality of the plated layer are achieved, the plated object is carefully removed from the plating bath. It may undergo additional post-treatment processes such as rinsing, drying, polishing, and coating to further enhance its appearance and properties.
Applications of Electroplating:
Electroplating finds widespread applications across various industries due to its ability to provide functional and decorative benefits to objects. Some notable applications include:
- Automotive Industry: Electroplating plays a crucial role in the automotive industry. It is used to plate various components such as trim, grilles, badges, and wheel rims. The process enhances the aesthetics of the vehicles and provides corrosion resistance, durability, and improved wear resistance.
- Electronics and Electrical Industry: Electroplating is extensively used in the electronics and electrical industry
Water Electrolysis:
Electrolysis is used in water electrolysis to produce hydrogen gas and oxygen gas. Water electrolysis is a chemical process that uses an electric current to split water molecules into hydrogen gas (H2) and oxygen gas (O2). It is a key method for producing hydrogen, a versatile and clean energy carrier. This article provides an in-depth overview of water electrolysis, including its principles, the electrolysis process, and its applications in various fields.
Principles of Water Electrolysis:
Water electrolysis is based on the principles of electrolysis, redox reactions, and the properties of water. It occurs in an electrolytic cell, which consists of two electrodes—an anode and a cathode—immersed in an electrolyte solution, typically water with an added electrolyte, such as sodium hydroxide (NaOH) or sulfuric acid (H2SO4).
- Anode: The anode in water electrolysis is typically made of an inert material, such as platinum or graphite. At the anode, the oxidation of water molecules takes place, resulting in the release of oxygen gas and the generation of positive hydrogen ions (H+).
- Cathode: The cathode is also made of an inert material. At the cathode, the reduction of water molecules occurs, leading to the production of hydrogen gas and the formation of hydroxide ions (OH–) from water molecules.
- Electrolyte Solution: The presence of an electrolyte in the water enhances its conductivity by providing ions for the movement of electric charge. It allows for the flow of current between the electrodes and facilitates the transport of ions during the electrolysis process.
- External Power Source: An external power source, such as a direct current power supply, is connected to the anode and cathode. It supplies the electric current necessary to drive the electrolysis reaction.
Water Electrolysis Process:
Water electrolysis involves several steps to separate water molecules into hydrogen and oxygen gases:
- Electrolyte Preparation: The electrolyte solution is prepared by adding a small amount of an electrolyte, such as sodium hydroxide or sulfuric acid, to water. This helps in improving the conductivity of water, allowing for the efficient flow of current during electrolysis.
- Electrolysis Cell Setup: The electrolyte solution is placed in an electrolysis cell, which consists of two compartments separated by an ion-permeable membrane or a salt bridge. The anode and cathode are immersed in the electrolyte solution, and the electrodes are connected to the external power source.
- Electrolysis Process: When the electric current is applied, the following reactions occur at the anode and cathode:
- Anode (Oxidation): At the anode, water molecules lose electrons and undergo oxidation. This results in the formation of oxygen gas (O2) and positive hydrogen ions (H+).
2H2O(l) -> O2(g) + 4H+(aq) + 4e-
- Cathode (Reduction): At the cathode, the positive hydrogen ions (H+) gain electrons and undergo reduction. This leads to the production of hydrogen gas (H2) and hydroxide ions (OH–).
4H+(aq) + 4e– -> 2H2(g) + 4OH-(aq)
- Overall Electrolysis Reaction: The overall reaction for water electrolysis is the combination of the anode and cathode reactions, resulting in the splitting of water molecules into hydrogen and oxygen gases.
2H2O(l) -> 2H2(g) + O2(g)
- Collection of Gases: The hydrogen gas and oxygen gas produced during electrolysis are collected
Top 10 books on electrolysis
Here are ten books that cover various aspects of electrolysis:
- “Electrochemical Methods: Fundamentals and Applications” by Allen J. Bard and Larry R. Faulkner: This comprehensive textbook covers the principles, techniques, and applications of electrochemical methods, including electrolysis.
- “Electrolysis and Electrochemical Engineering” by C. N. Satterfield: This book provides a thorough introduction to electrolysis and electrochemical engineering, covering fundamental principles, processes, and practical applications.
- “Electrochemical Engineering: Principles” by Geoffrey Prentice: This book focuses on the principles of electrochemical engineering, including electrolysis, and covers topics such as electrode kinetics, mass transport, and reactor design.
- “Electrolysis: Theory, Types and Applications” by Thomas W. Bond: This book offers a comprehensive overview of electrolysis, discussing its theory, different types of electrolytic cells, and applications in various industries.
- “Electrolysis and Water” by Friedrich Huggenberger: This book explores the application of electrolysis in water treatment, covering topics such as electrochemical disinfection, water purification, and desalination.
- “Electrolysis: Principles and Techniques” by Paolo M. O. Herrera and Carlos V. C. Geraldes: This book provides a detailed examination of the principles and techniques of electrolysis, including experimental methodologies and practical applications.
- “Electrolysis: Energy Storage and Conversion” edited by Olaf Conrad: This book focuses on the role of electrolysis in energy storage and conversion, discussing topics such as water splitting, fuel cells, and electrochemical energy storage systems.
- “Industrial Electrochemistry” by Derek Pletcher: This book covers the industrial applications of electrochemistry, including electrolysis processes used in chemical synthesis, metal extraction, and electroplating.
- “Electrochemistry for Chemists” by Robert J. Gale: This book provides a comprehensive introduction to electrochemistry, including electrolysis, for chemists and chemical engineers, with an emphasis on practical applications.
- “Electrodeposition: The Materials Science of Coatings and Substrates” by Frank C. Walsh: This book focuses on the electrodeposition process, including electroplating, and its application in coating materials onto substrates, covering topics such as process control and coating properties.
These books cover a wide range of topics related to electrolysis, including fundamental principles, experimental techniques, practical applications, and specific areas of interest within the field. Whether you are a student, researcher, or professional working with electrolysis, these books can provide valuable insights and knowledge.